Hernia patch solid-supported with antibiotic and preparation method
A technology of antibacterial drugs and patches, applied in antibacterial drugs, medical science, powder delivery, etc., to achieve high drug loading, mature technology, and non-immunogenic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Norvancomycin microspheres were prepared by double emulsion solvent evaporation method. Aqueous norvancomycin hydrochloride (20 mg / ml) was used as the inner aqueous phase; PLGA was dissolved in CH 2 Cl 2 Forming a concentration of 1% PLGA solution as the oil phase; adding the inner water phase to the oil phase at a volume ratio of 1:6, at room temperature, a high-pressure homogenizer is homogenized at a speed of 5000rpm for 30s to form colostrum (W / O); PVA (PVA 05-88, the same below) is used as an emulsifier, and the concentration of 1% PVA aqueous solution is prepared as the external water phase; the colostrum is poured into the external water phase, and the volume ratio of the colostrum to the external water phase is 1: 3. Form double emulsion (W / O / W), stir at room temperature with a constant speed mixer at 200rpm for 2h, volatilize the organic solvent, centrifuge, wash, freeze (speed 1000rpm, centrifuge for 10min, gradient cooling and drying at 20°C for 2h, 10°C for...
Embodiment 2
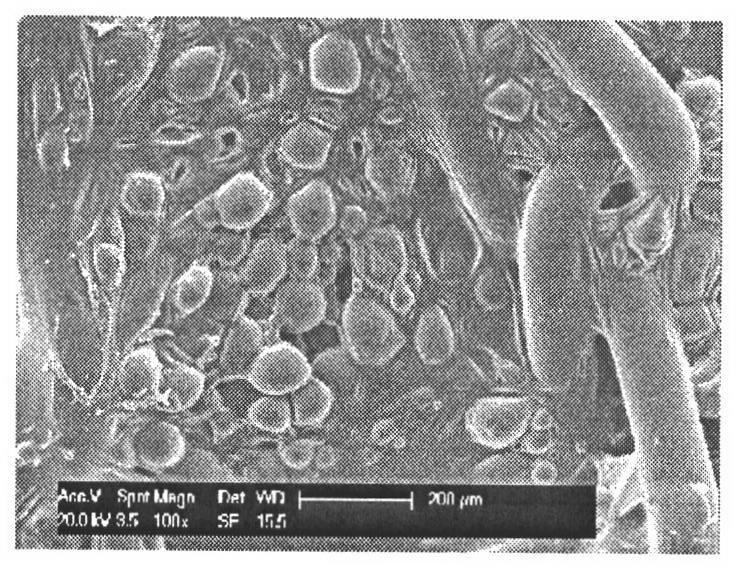
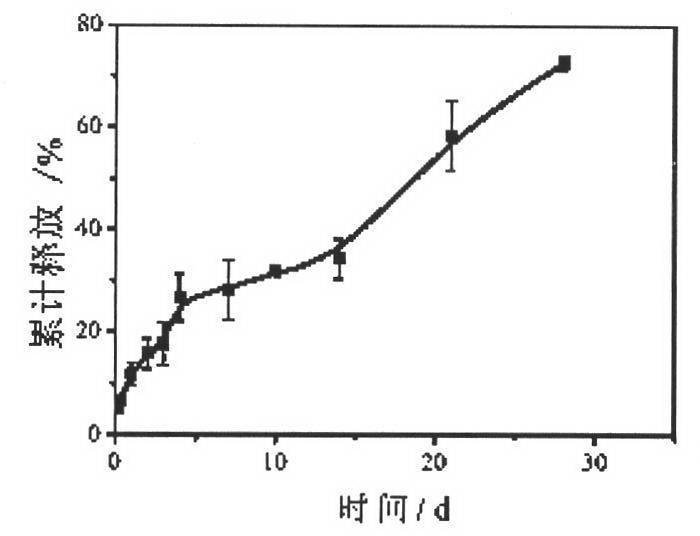
[0072] Cut the blank patch (polypropylene patch PCDM 1.01, Ethicon, U.S.) into a square, place it in 1% EVA cyclohexane solution for full infiltration, accurately weigh 30 mg of norvancomycin microspheres, and place the drug-loaded microspheres The balls were evenly dispersed on the patch, placed to dry, and the surface of the patch containing norvancomycin microspheres was obtained to be smooth, and the microspheres and the patch were not damaged, as shown in Fig. figure 2 . The accumulative release rate reaches 72.6%, and it can be released continuously for more than 28 days. It has obvious slow-release effect, such as image 3 . Forty SD rats were randomly divided into two groups, 20 in each group; they were the experimental group and the control group. After intraperitoneal injection of pentobarbital sodium anesthetized the two groups of rats, a longitudinal incision about 1 cm long was made in the middle of the back to reach the subcutaneous layer and free the subcutan...
Embodiment 3
[0074] Cut the polypropylene patch (polypropylene patch PCDM 1.01, Ethicon, USA) into a 1cm×1cm square, smear 0.25% EVA (FM 114.1, d 0.941) cyclohexane solution on the surface, volatilize at room temperature, and wait until it is completely When drying, accurately weighed norvancomycin hydrochloride (NV) is uniformly dispersed on its surface, and then the cyclohexane solution of EVA is put into a polytetrafluoroethylene tank by volatile casting method, and placed at room temperature to dry ) prepared EVA film, covering the surface ( Figure 8 ). The in vitro release results show that the cumulative release rate of the drug-loaded patch reaches 90.6%, and it can be released continuously for more than 24 hours, and has obvious sustained release effect (such as Figure 9 ).
[0075] Twelve Wistar rats were randomly divided into two groups, 6 in each group; they were the experimental group and the control group, respectively. After intraperitoneal injection of pentobarbital sod...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| mass | aaaaa | aaaaa |
| mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com