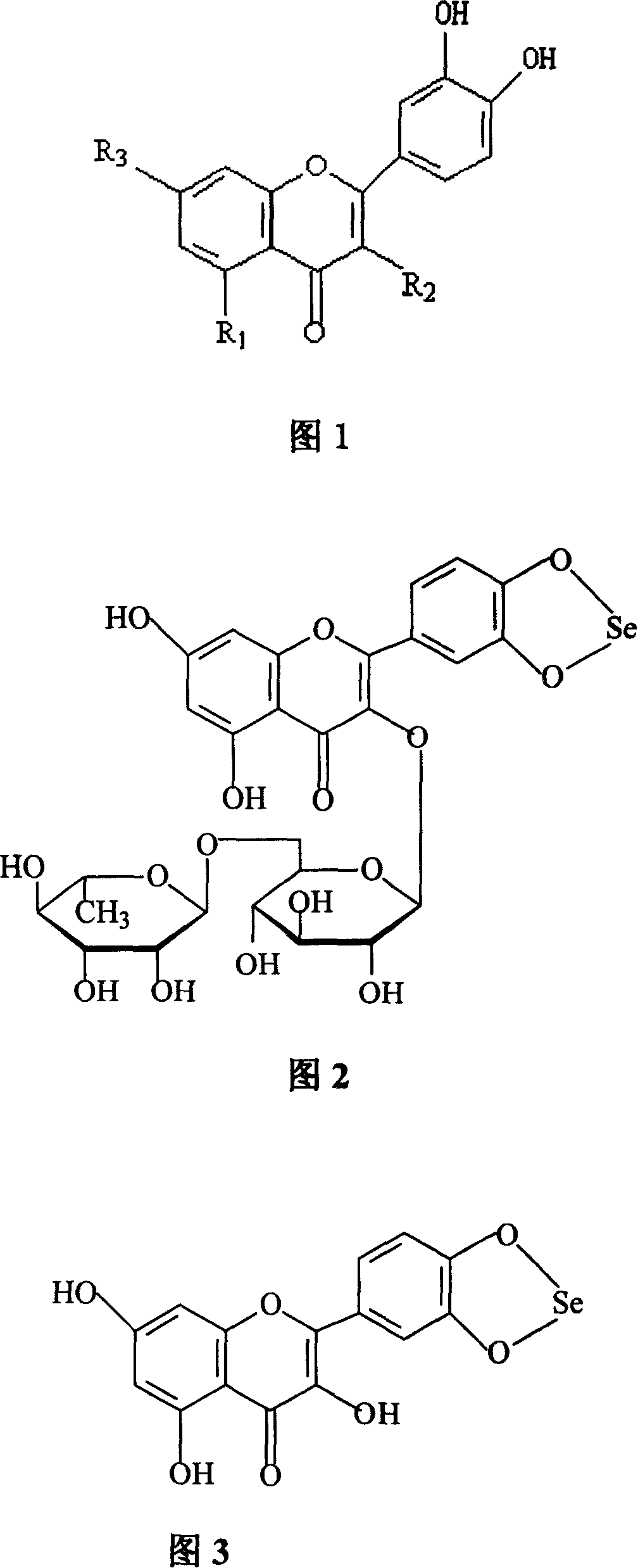
Method for preparing o-dihydroxyflavone-selenium complexes and medical use
A technology of dihydroxyflavonoids and complexes, which is applied in the fields of natural medicinal chemistry, drug synthesis and pharmacology, and can solve problems such as anti-tumor activity research that has not been reported yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Embodiment 1: the preparation of rutin-selenium complex
[0015] Add 1 g (0.001641 mol) of rutin into dry 20 ml of pyridine, stir, drop into 0.5 ml of selenium oxychloride, dry it, and react at room temperature for 48 hours, add ether to precipitate a precipitate, wash repeatedly with ether and chloroform to obtain a precipitate, Vacuum freeze-drying to obtain a brown rutin-selenium complex. Weighed 1.332g, yield 51.5%. The newly synthesized compounds were identified by various physical methods, including 1 HNMR, MS and elemental analysis. Its structure is as follows (see accompanying drawing 2 of specification sheet):
[0016] Figure 2 Rutin-selenium complex
[0017] mp: 185.0-189.0℃.IR(KBr)3219cm -1 (O-H), 1654cm -1 (C=O), 1610, 1537cm -1 (C=C), 1198cm -1 (C-O-C), 750cm -2 (C-H), 680cm -2 (Se-O). 1 H NMR (CDCl 3 , 300MHz), δ (ppm): 11.68 (s, 1H, 5-OH), 10.56 (s, 1H, 7-OH), 7.53 (d, 1H, 2'-H), 7.38 (d, 1H, 5 '-H), 6.70 (d, 1H, 6'-H), 6.13 (d, 1H, 8-H), 5.9...
Embodiment 2
[0018] Embodiment 2: the preparation of quercetin-selenium complex
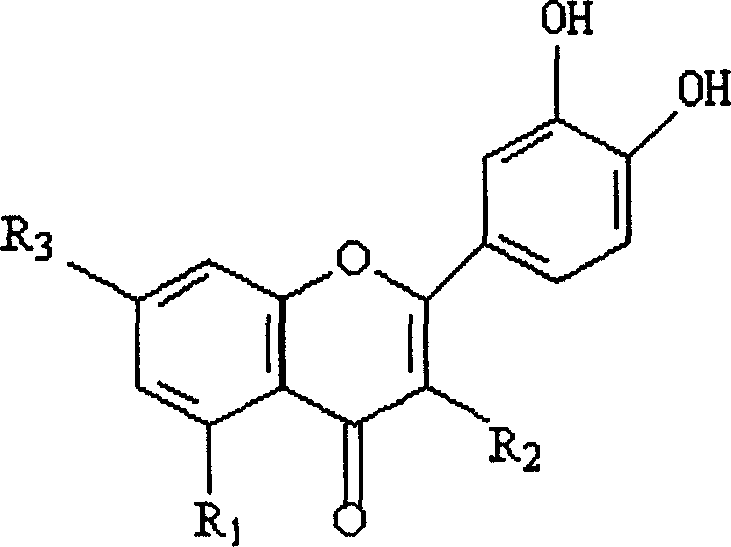
[0019] Add 2g (0.006623mol) of quercetin into dry 30ml of pyridine, stir, drop into 2.0ml of selenium oxychloride, dry, and react at room temperature for 48h, add ether to precipitate a precipitate, wash repeatedly with ether and chloroform to obtain a precipitate , vacuum freeze-drying, and finally a brown quercetin-selenium complex. Weighed 2.437g, yield 47.8%. Its structure is as follows (see accompanying drawing 3 of specification sheet):
[0020] Figure 3 Quercetin-selenium complex
[0021] mp: 315.0-318.0℃.IR(KBr) 1651cm -1 (C=O), 1606, 1530cm -1 (C=C), 1206cm -1 (C-O-C), 755cm -1 (C-H), 680cm -1 (Se-O). 1 H NMP (CDCl 3 , 300MHz), δ (ppm) 11.88 (s, 1H, 5-OH), 10.50 (s, 1H, 7-OH), 10.26 (s, 1H, 3-OH), 7.60 (d, 1H, 2'- H), 7.29(d, 1H, 5'-H), 6.71(d, 1H, 6'-H), 6.18(d, 1H, 8-H), 5.98(d, 1H, 6-H), MS (m / z): 378.1 (M - +Se). Molecular formula: C 15 h 8 o 7 Se, elemental analysis: Calcd for (%)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com