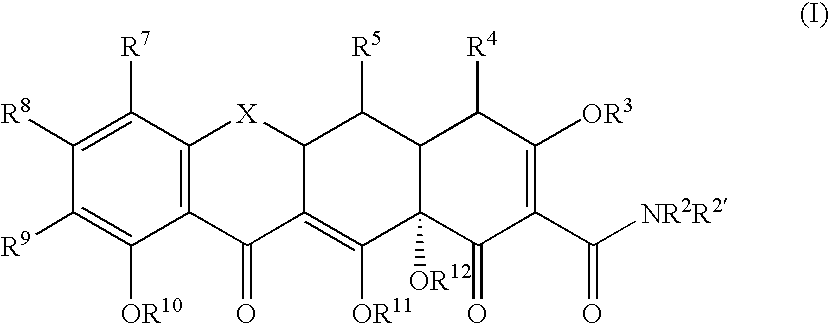
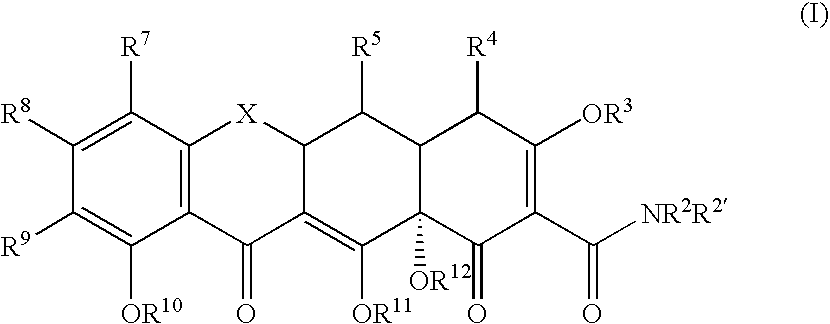
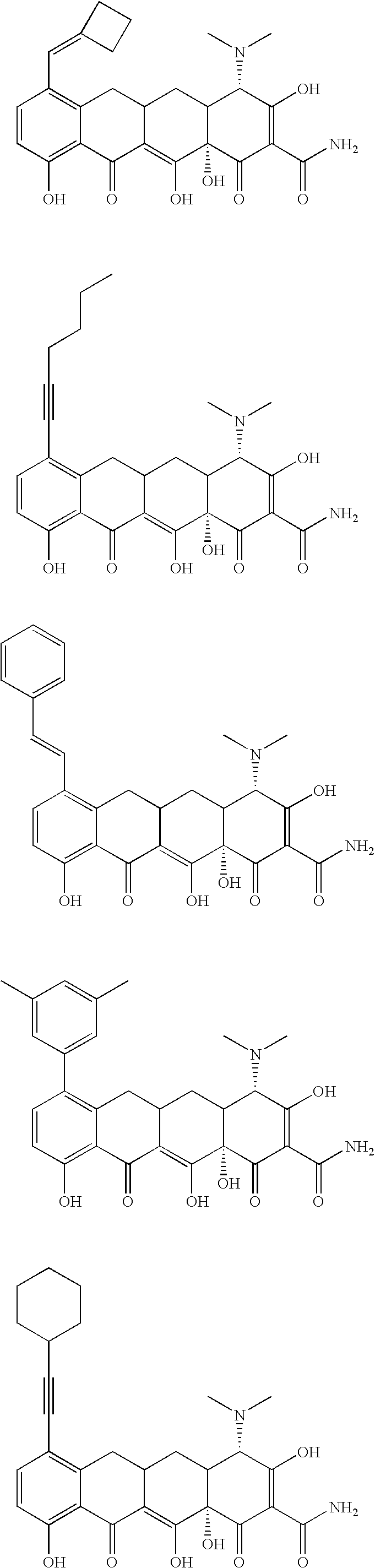
Substituted tetracycline compounds as antifungal agents
a technology of substituted tetracycline and antifungal agent, which is applied in the direction of tetracycline active ingredients, biocide, drug compositions, etc., can solve the problems of adverse effects, high morbidity and mortality of opportunistic systemic fungal infections, and increase the incidence of the infection, so as to achieve broad antifungal spectrum of activity, high morbidity and mortality, and the effect of increasing the inciden
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0136] Synthesis of Tetracycline Compounds
[0137] The following example discusses methods of synthesizing the substituted tetracycline compounds of the invention. One of ordinary skill in the art will be able to use the presented examples and / or art recognized techniques to synthesize the compounds of the invention.
[0138] Experimental
[0139] Melting points were taken on a Mel-Temp capillary melting point apparatus and are uncorrected. Nuclear magnetic resonance (1H NMR) spectra were recorded at 300 MHz on a Bruker Avance spectrometer. The chemical shift values are expressed in δ values (ppm) relative to tetramethylsilane or 3-(trimethylsilyl)-1-propanesulfonic acid, sodium salt, as either an internal or external standard using CDCl3, DMSO-d6, or MeOH-d4 as the solvent. Column chromatography was performed according to the method of Still using Baker “flash” grade silica gel (40 μm) that was treated with a saturated solution of Na2EDTA, washed with water, filtered and dried in an ove...
example 2
[0153] Antifungal Activity of Substituted Tetracycline Compounds
[0154] Antifungal activity of the tetracyclines was determined by a broth microdillution technique following NCCLS (1997) Standards. Assays were setup using a Tecan Genesis robotic workstation. All drugs were dissolved in 10% DMSO. Drug concentration ranged from 0.125 to 64 μg / mL in 2 fold serial dilutions. Each tetracycline was tested at 10 concentrations ranging from 0.125 to 64 μg / mL. The compounds were tested for their antifungal activity against Candida albicans (ATCC#90028). The final concentration of DMSO was kept below 1%. Checkerboard analysis of the initial hits will be performed to better determine the activity of the compound.
[0155] The strains tested include those listed in Table 1.
TABLE 1GenusSpeciesATCC / FGSC #AspergillusfumigatusATCC 13073 (Fresenius)AspergillusnidulansFGSCA991 (wt)CandidaalbicansATCC90028CandidaalbicansPCI-1CandidaalbicansPCI-17CandidaalbicansATCC 36082CandidaglabrataATCC 90030Candid...
example 3
[0157] Mammalian Cytotoxicity Assay
[0158] COS-1 and CHO cell suspensions are prepared, seeded into 96-well tissue culture treated black-walled microtiter plates (density determined by cell line), and incubated overnight at 37° C., in 5% CO2 and approximately 95% humidity. The following day serial dilutions of drug are prepared under sterile conditions and transferred to cell plates. Cell / Drug plates are incubated under the above conditions for 24 hours. Following the incubation period, media / drug is aspirated and 50 μl of Resazurin is added. Plates are then incubated under the above conditions for 2 hours and then in the dark at room temperature for an additional 30 minutes. Fluorescence measurements are taken (excitation 535 nm, emission 590 nm). The IC50 (concentration of drug causing 50% growth inhibition) is then calculated. The cytotoxcity of both unsubstituted minocycline and doxycycline were found to be greater than 25.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com