Fluid-assisted medical devices, systems and methods
a technology of medical devices and fluids, applied in the field of fluid-assisted medical devices, systems and methods, electrosurgical, can solve the problems of tissue desiccation, tissue sticking to electrodes, tissue perforation,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
Throughout the present description, like reference numerals and letters indicate corresponding structure throughout the several views, and such corresponding structure need not be separately discussed. Furthermore, any particular feature(s) of a particular exemplary embodiment may be equally applied to any other exemplary embodiment(s) of this specification as suitable. In other words, features between the various exemplary embodiments described herein are interchangeable as suitable, and not exclusive.
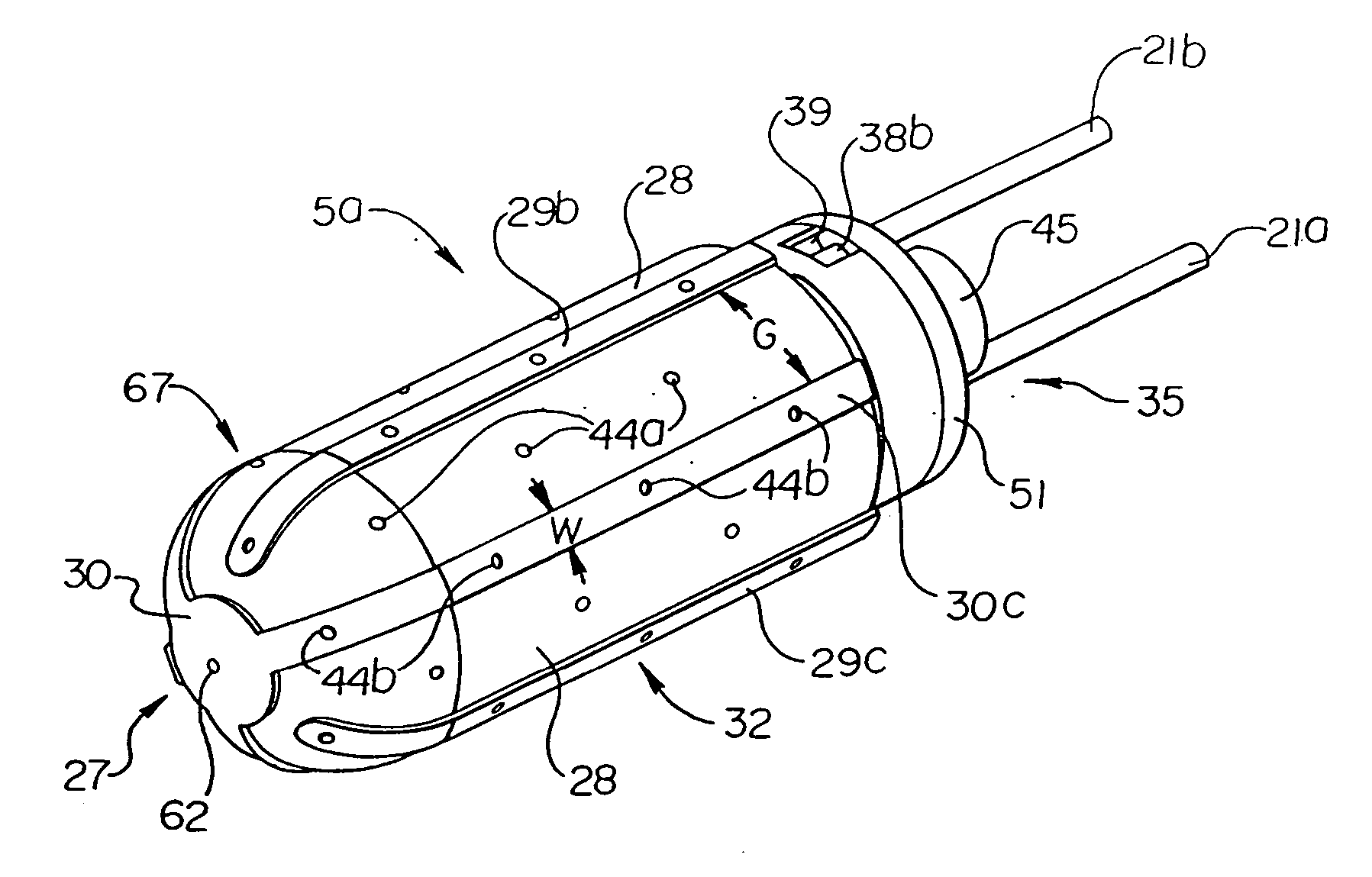
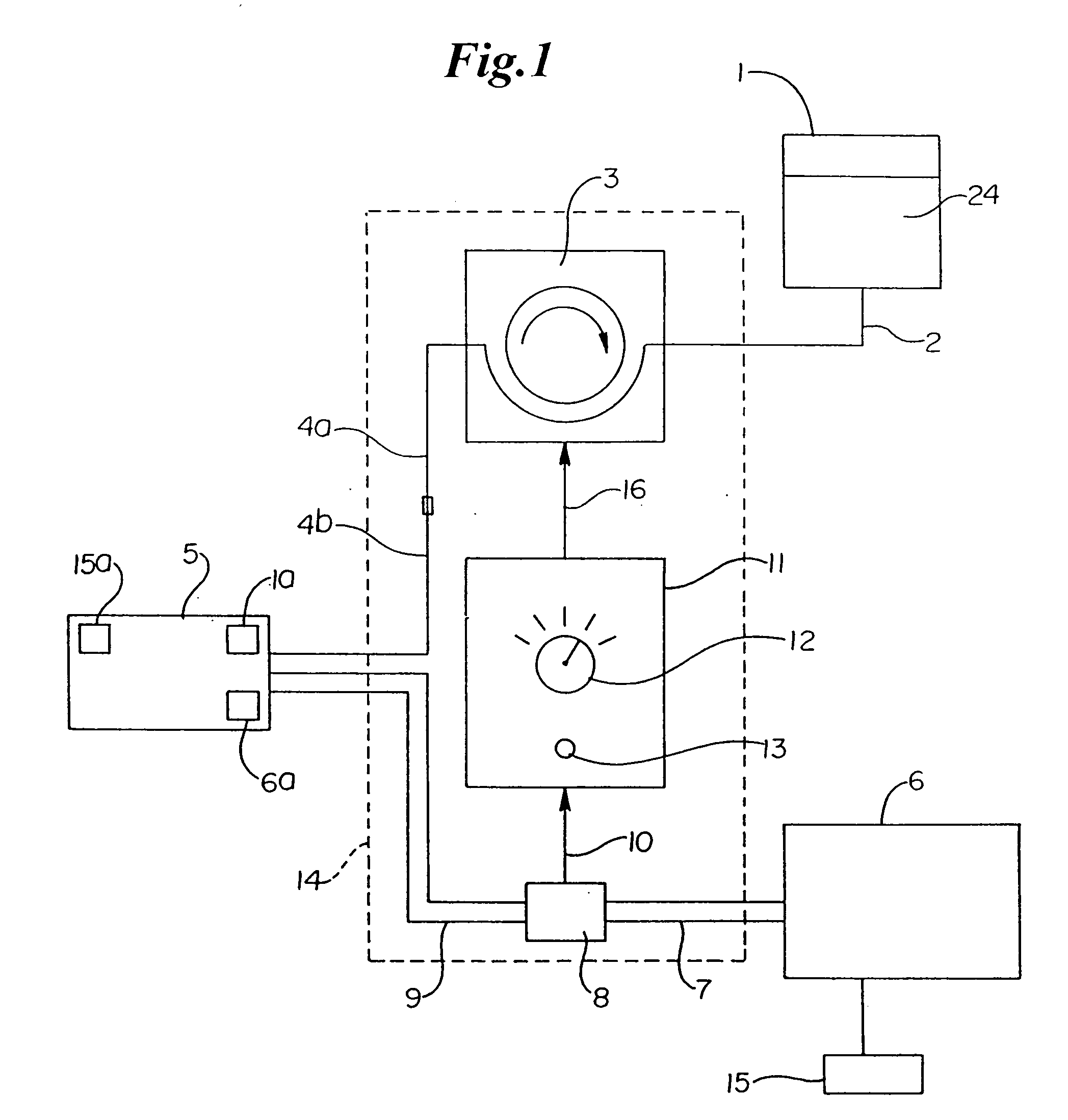
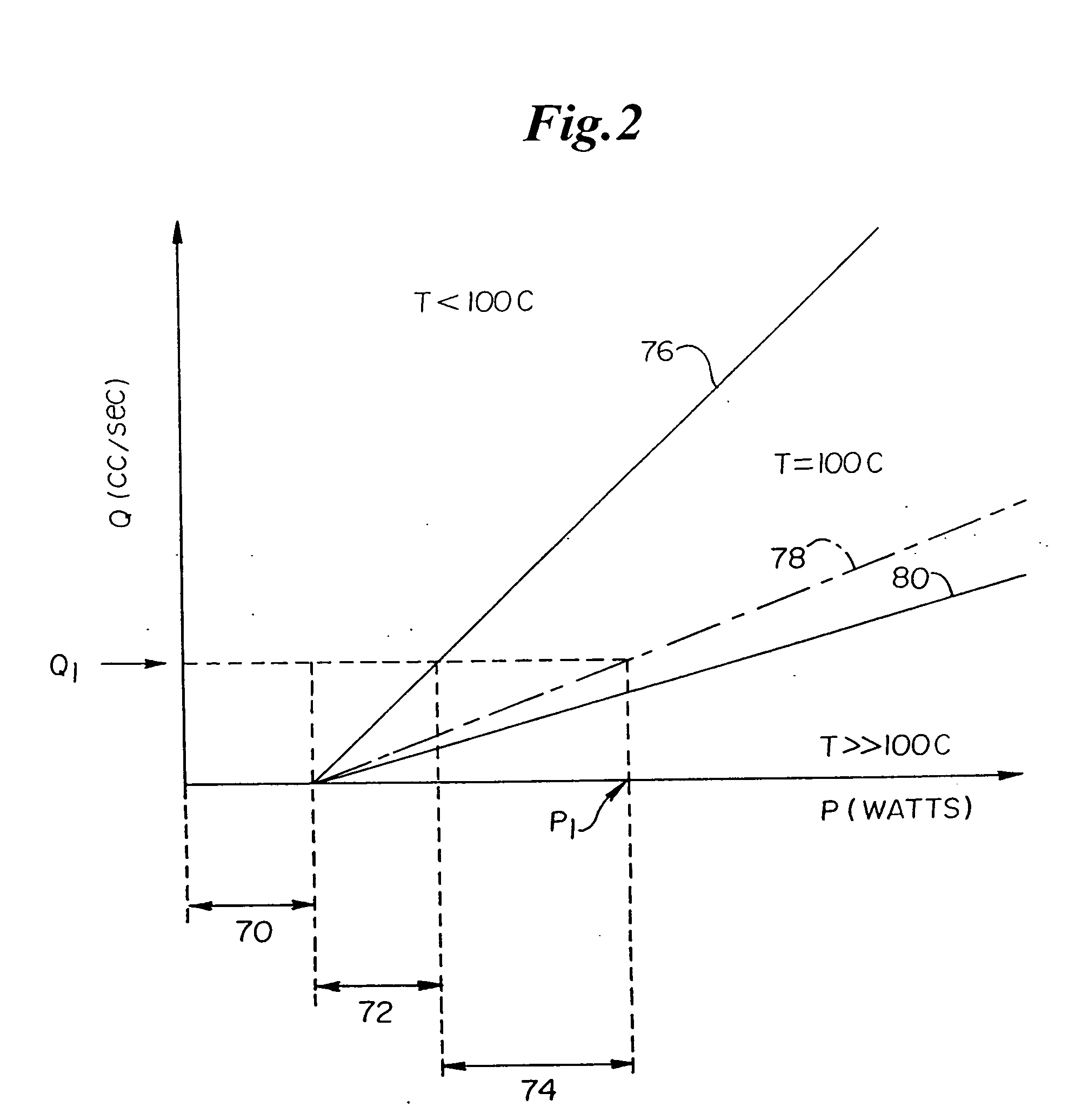
The invention provides systems, devices and methods that preferably improve control of tissue temperature at a tissue treatment site during a medical procedure. The invention is particularly useful during surgical procedures upon tissues of the body, where it is desirable to shrink tissue, coagulate fluids (e.g. oozing blood), and at least partially occlude lumens, vessels (e.g. lumen of blood vessels (e.g. arteries, veins), intestines (e.g. absorbent vessels)) and airways (e.g. tr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com