Pharmaceutical composition containing celecoxib and tramadol
A composition and pharmacy technology, applied in the field of tramadol's pharmacy composition, can solve the problems of poor pain relief and other problems, and achieve the effects of easy packaging, suitable for large-scale production, excellent hardness and interlayer adhesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0080] Hereinafter, the present invention will be illustrated by way of examples. On the other hand, it should be noted that these examples do not limit the invention in any way.
[0081][Basic conditions]
[0082] The celecoxib used in this example is a raw material containing D90 of 10 to 15 μm and D50 of 4 to 7 μm.
[0083] "200 mg celecoxib capsules" and "100 mg tramadol hydrochloride (Tridol) sustained-release tablets" refer to single doses of celecoxib and tramadol on the market, respectively.
[0084] The approximate main components of the product names used in this Example are as follows.
[0085] Table 1
[0086]
[0087]
Embodiment 1
[0088] [Example 1] Formulation of insoluble drugs after compatibilization
[0089] Example 1-1) Formulation using water-soluble polymers, surfactants, and sugars—Confirmation of the dissolution form according to the ratio of water-soluble polymers and surfactants: using celecoxib and the following excipients, with The wet granule form was combined, dried, granulated, blended and purified and subjected to in vitro dissolution testing.
[0090] Table 2
[0091]
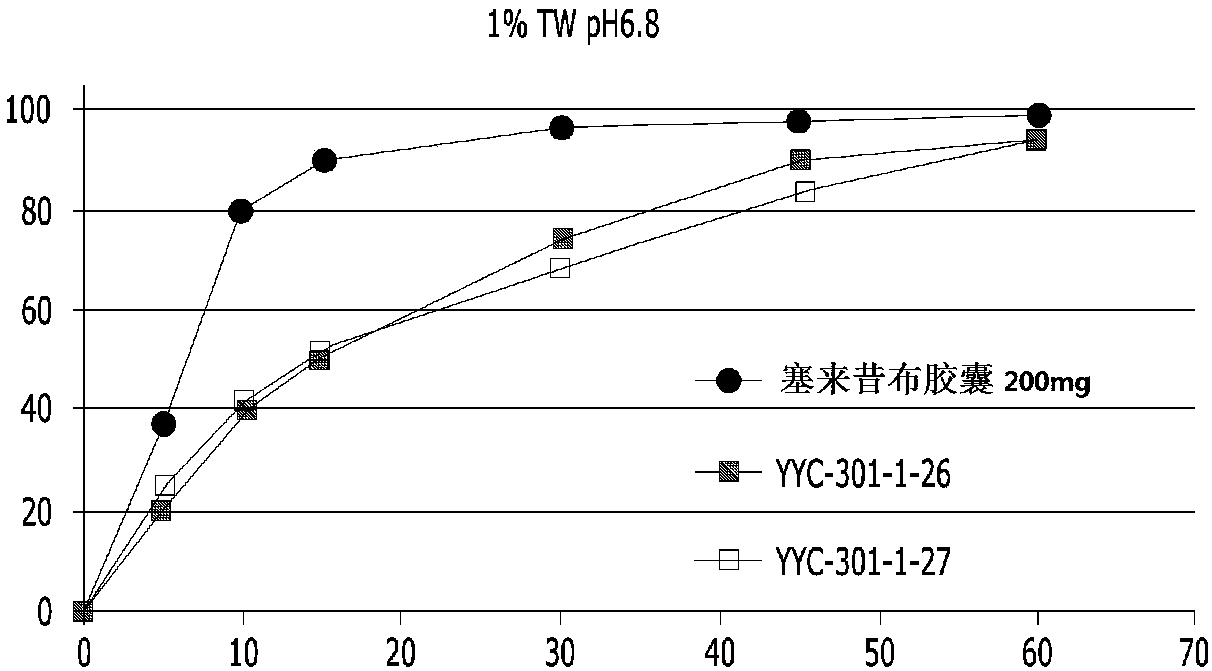
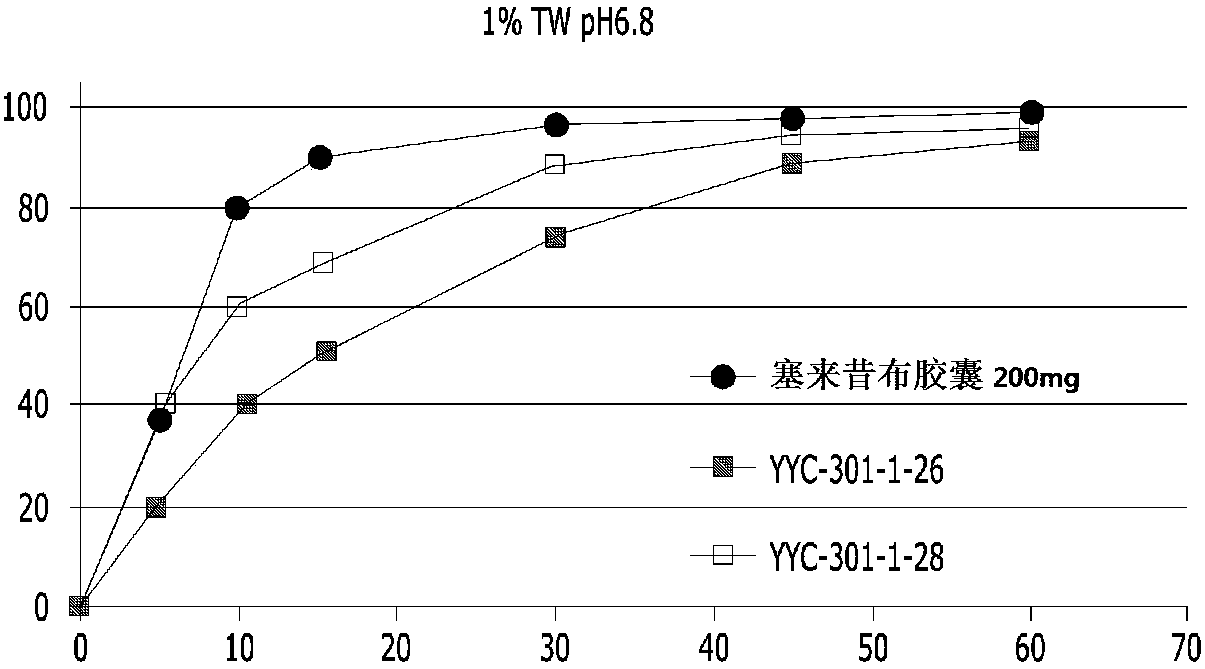
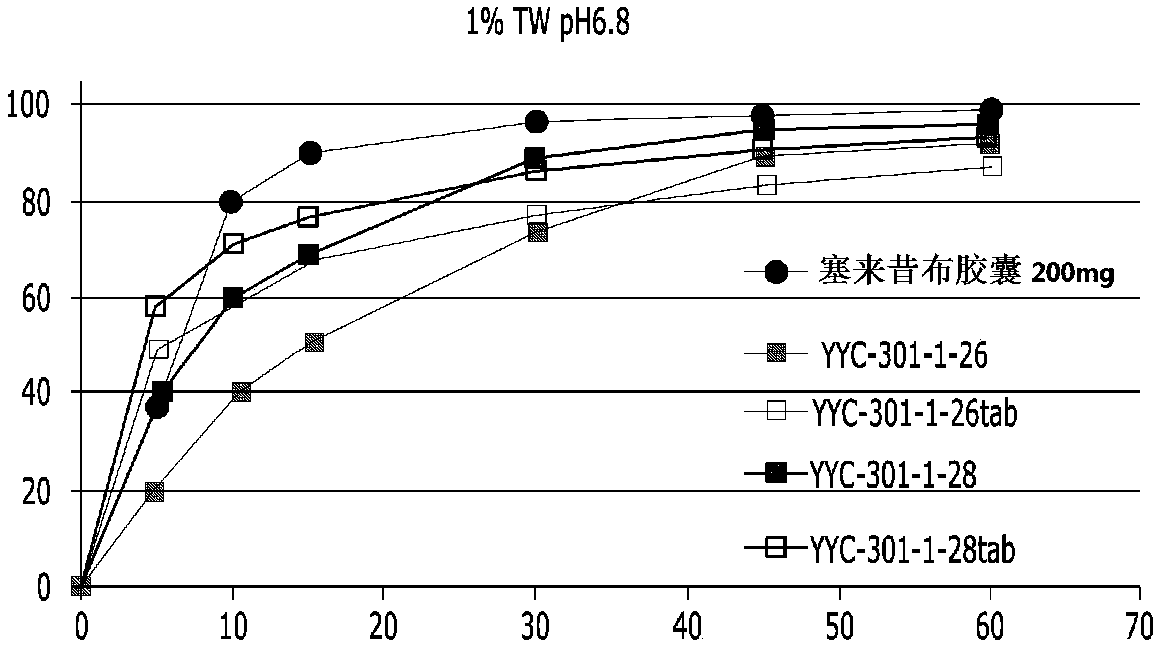
[0092] Only the celecoxib granules (granules) of Table 2 are filled in the capsules, and the dissolution evaluation is carried out. The results are as follows in Table 3 and figure 1 .
[0093] table 3
[0094]
[0095] Example 1-2) Formulation using water-soluble polymers, surfactants, and saccharides—confirmation of the dissolution form according to the amount of saccharides: Combine celecoxib and the following excipients in wet granule form Dried, granulated, blended and purified, and subjected to in vitro...
Embodiment 2
[0128] [Example 2] Sustained release of water-soluble drugs
[0129] YYC-301-1-(59+XX) in the following table refers to the bilayer tablet laminated with the celecoxib domain YYC-301-1-59 of Table 25 and the tramadol domain YYC-301-1-XX .
[0130] Example 2-1) Formulation using Ethylcellulose and wax-like lipid excipients—confirmation of poor dissolution form according to the amount of wax-like lipid excipients: using tramadol hydrochloride and the following excipients formulations, combined, dried, granulated, blended and purified in wet granule form, and subjected to in vitro dissolution testing.
[0131] Table 15
[0132]
[0133] The stripping evaluation result of table 15 is as follows table 16 and Figure 8 shown.
[0134] Table 16
[0135]
[0136] Example 2-2) Formulation using ethyl cellulose and wax-like lipid excipients—confirmation of poor dissolution morphology according to the amount of ethyl cellulose: using tramadol hydrochloride and the following ex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com