Methods and implantable devices and systems for long term delivery of a pharmaceutical agent
a technology of implantable devices and pharmaceutical agents, applied in the field of drug delivery, can solve the problems of unsatisfactory side effects, inability to administer iv for a long time, and infection such a significant risk,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
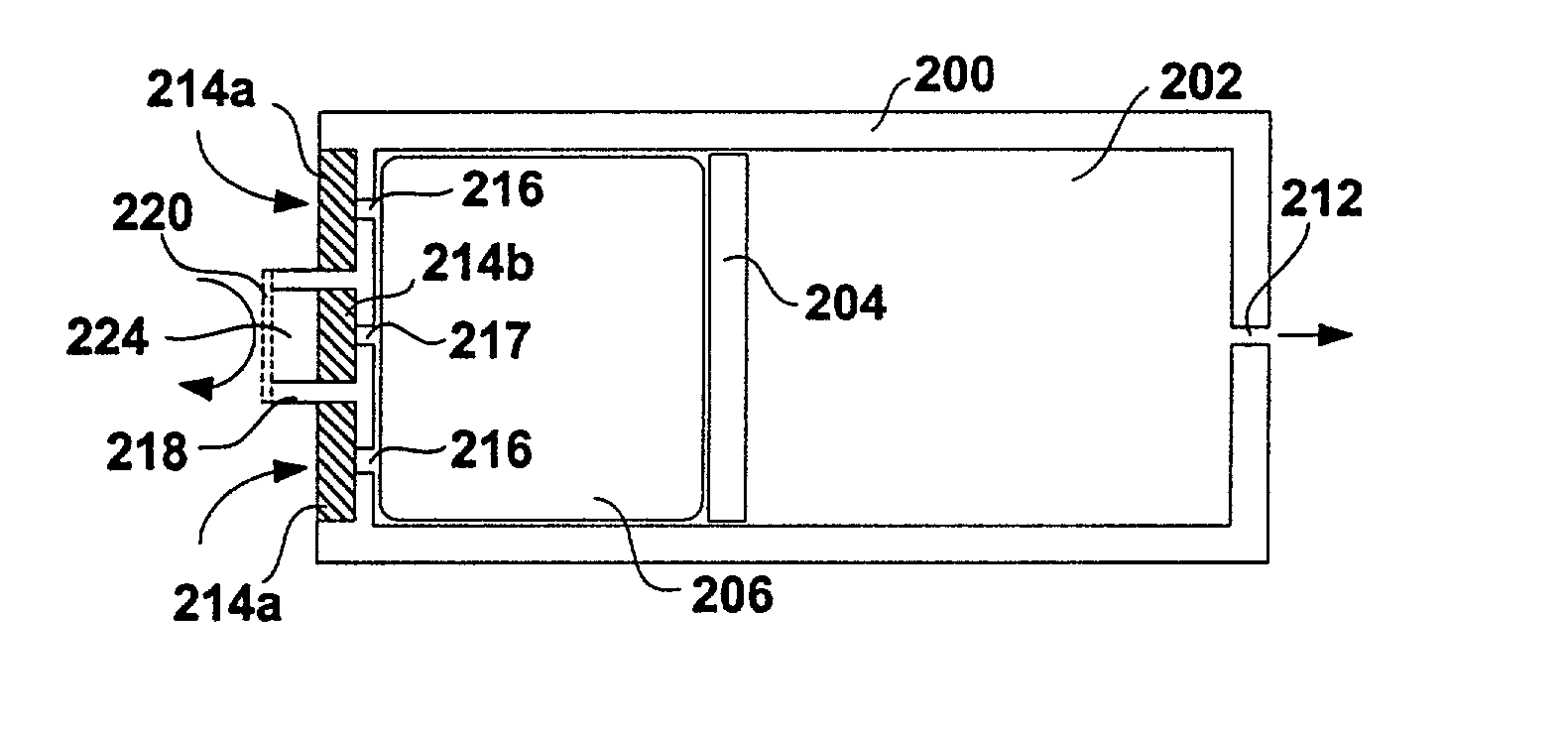
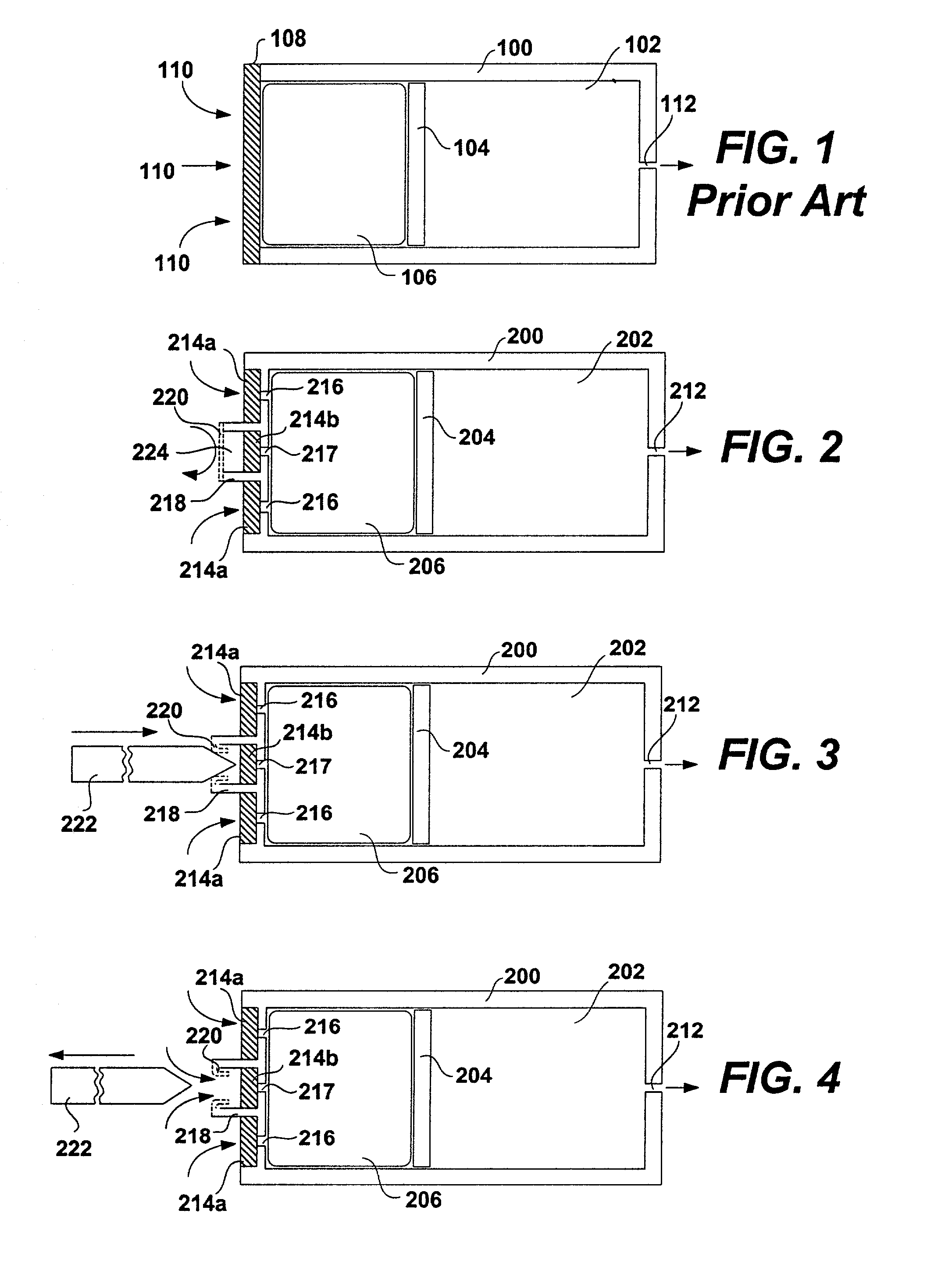
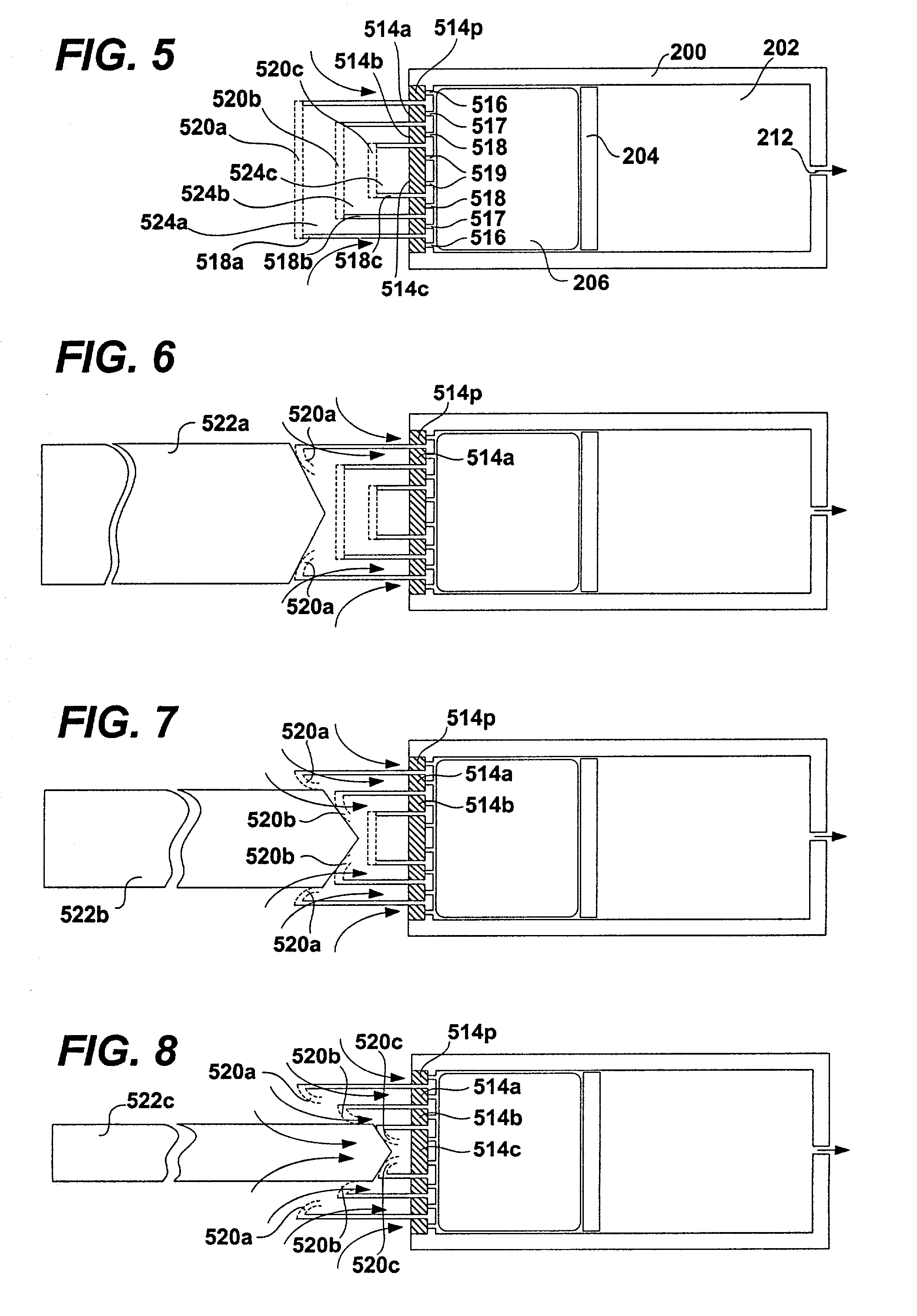
[0049] FIG. 1 shows a schematic diagram of a conventional osmotic pump. The pump includes a housing 100. The housing 100 may be shaped as a cylinder and may be divided into a drug reservoir 102 and an osmotic engine compartment 106. A piston 104 separates the drug reservoir 102 and the osmotic engine compartment 106. The movement of the piston 104 toward the delivery orifice 112 provides the driving force to effuse the drug contained within the drug reservoir 102. A semi permeable membrane 108 is disposed at one end of the pump, covering the opening thereof opposite the delivery orifice 112. The semi permeable membrane 108 is permeable to water. Therefore, when the pump is placed within the patient's body or other aqueous medium, water tends to cross the semi permeable membrane 108 into the osmotic engine compartment 106. The osmotic engine within the compartment 106 is the driving force that maintains the solution inside the pump (but outside the reservoir 102) at a fully saturated...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com