Pulmonary delivery of spherical insulin microparticles
A technology of insulin and spherical particles, which can be used in drug combination, drug delivery, pharmaceutical formulation, etc., to solve the problems of poor patient compliance and shortness of breath.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0214] General method for preparing small spherical particles of insulin
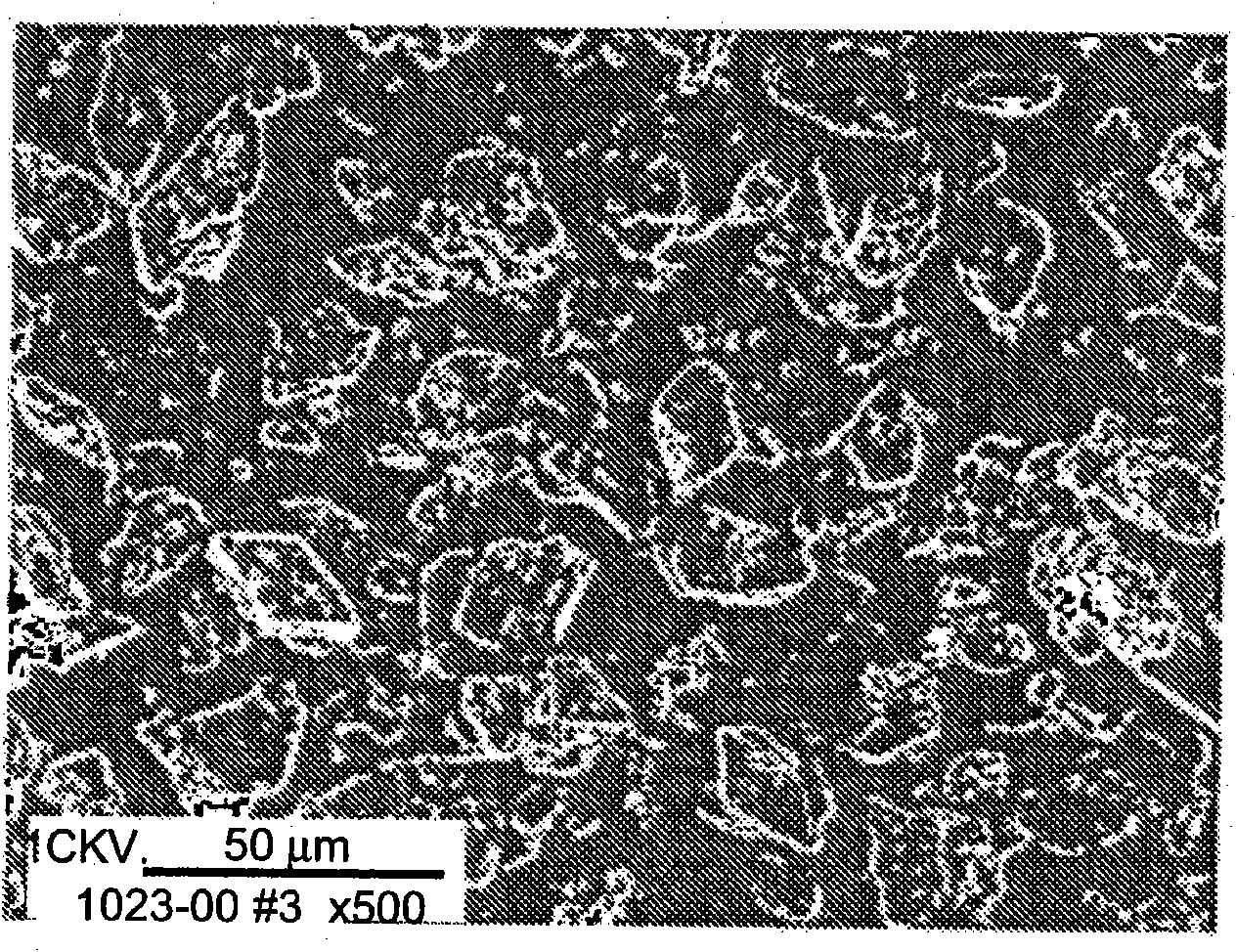
[0215] A solution buffered at pH 5.65 (0.033M sodium acetate buffer) containing 16.67% PEG 3350 was prepared. A concentrated slurry of crystalline insulin zinc was added to the solution with stirring. The concentration of insulin in the final solution was 0.83 mg / mL. The solution was heated to about 85 to 90°C. Insulin crystals dissolve completely within 5 minutes in this temperature range. When the temperature of the solution is lowered at a controlled rate, small spherical particles of insulin begin to form around about 60°C. Yield increased with increasing PEG concentration. The process yielded small spherical particles with various size distributions, with an average size of 1.4 μm.
[0216] The resulting insulin spheroids were separated from PEG by washing the spheres by diafiltration under conditions in which the spheroids do not dissolve. Use containing Zn 2+ aqueous solution, the insulin ...
Embodiment 2
[0217] Example 2: A non-stirring batch process for the manufacture of small spherical insulin particles
[0218] Suspend 20.2 mg of crystalline insulin zinc in 1 mL of deionized water at room temperature. Add 50 microliters of 0.5N HCl to the insulin. Add 1 mL of deionized water to form a 10 mg / mL solution of crystalline insulin zinc. 12.5 g polyethylene glycol 3350 (Sigma) and 12.5 g polyvinylpyrrolidone (Sigma) were dissolved in 50 mL of 100 mM sodium acetate buffer, pH 5.7. Adjust the volume of the polymer solution to 100 mL with sodium acetate buffer. To the 800 µl polymer solution in a microcentrifuge tube, add 400 µl 10 mg / mL insulin solution. When mixed, the insulin / polymer solution became cloudy. A control was prepared using water instead of the polymer solution. Heat the microcentrifuge tube in a water bath at 90 °C for 30 min without mixing or agitation, then remove and place on ice for 10 min. The insulin / polymer solution was clear after removal from the 90°C ...
Embodiment 3
[0219] Example 3: Continuous flow-through process for the manufacture of small spherical insulin particles
[0220] Weigh out 36.5 mg of insulin and suspend in 3 mL of deionized water. Add 30 µL of 1N HCl to dissolve the insulin. Adjust the final volume of the solution to 3.65 mL with deionized water. 7.3 mL of PEG / PVP solution (25% PEG / PVP, pH 5.6 in 100 mM NaOAc buffer) was then added to the insulin solution to give a final total volume of 10.95 mL of insulin solution. The solution was then vortexed, resulting in a homogeneous suspension of insulin and PEG / PVP.
[0221] Pass the insulin suspension through Tubing (TFE 1 / 32 inch inner diameter elastic tubing) was connected to a BioRad peristaltic pump running at 0.4 mL / min. The tubing from the pump was submerged in a water bath maintained at 90°C before being inserted into a collection tube soaked in ice. When the temperature of the insulin solution was lowered from about 90°C in a water bath to about 4°C in a collection...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com