Preparation and application of glucose-sensitive insulin dosing system
A glucose-sensitive, drug delivery system technology, applied in the field of preparation of a new glucose-sensitive insulin drug delivery system, can solve problems restricting clinical applications, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
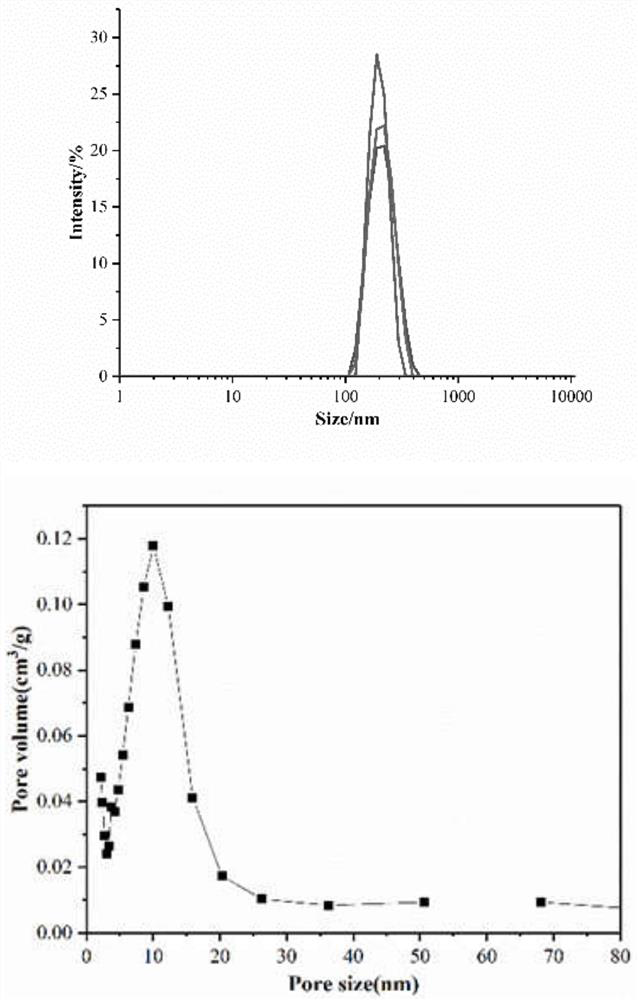
[0060] The preparation of example 1 mesoporous silica
[0061] sample 1
[0062] Weigh 0.5g CTAB and dissolve it in 500mL double-distilled water, stir it at 600rpm for 0.5h in a water bath at 80°C to make it fully dissolved, add 150mL n-octane dropwise to the above clear solution, connect the condensing reflux device , and continued to stir for 2h to obtain a uniform emulsion. Slowly add 26 mL of styrene monomer dropwise into the emulsion. Under nitrogen protection, 0.075g of lysine, 5.0mL of tetraethyl orthosilicate, and 0.125g of azobisisobutylamidine hydrochloride were sequentially added to the reaction solution. After reacting for 2 hours, the suspension was cooled naturally, left to stand for about 10 hours, an equal volume of absolute ethanol was added, centrifuged at 10000 rpm for 8 minutes, and the precipitate was washed 3 times with absolute ethanol. After the precipitate was dried at 60°C for 12h, it was calcined at 550°C for 6h to remove the pore-enlarging agent ...
example 2
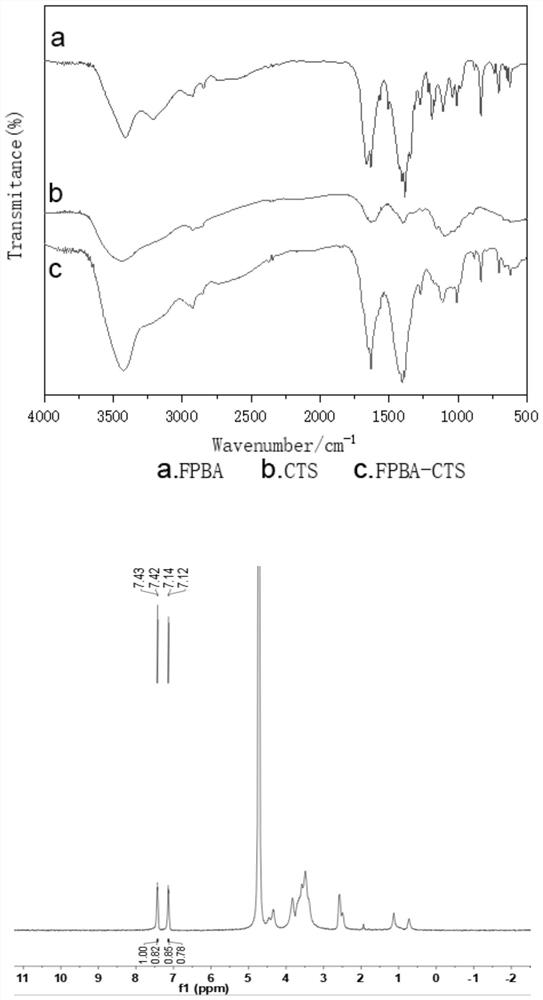
[0067] Example 2 Preparation of 4-formylphenylboronic acid grafted chitosan glucose sensitive material
[0068] sample 1
[0069] Weigh 5 mg of 4-formylphenylboronic acid and dissolve it in 0.5 mL of methanol, weigh 10 mg of chitosan CTS (10kD) and dissolve it in 7 mL of 1% acetic acid solution, mix the two solutions, react for 2 hours, and weigh sodium borohydride 7.2mg was added to the above mixed solution, the reaction was stirred at 25°C for 24 hours, centrifuged at 10000rpm for 5min and washed with methanol, ethanol and water, and the polymer of the obtained 4-formylphenylboronic acid grafted chitosan was freeze-dried, and Store at 2-8°C until use (FPBA-CTS).
[0070] sample 2
[0071] Weigh 2.5mg of 4-formylphenylboronic acid and dissolve it in 0.5mL of methanol, weigh 10mg of chitosan (50kD) and dissolve it in 7mL of 1% acetic acid solution, mix the two solutions, react for 2 hours, and weigh sodium borohydride 7.2mg was added to the above mixed solution, the reactio...
example 3
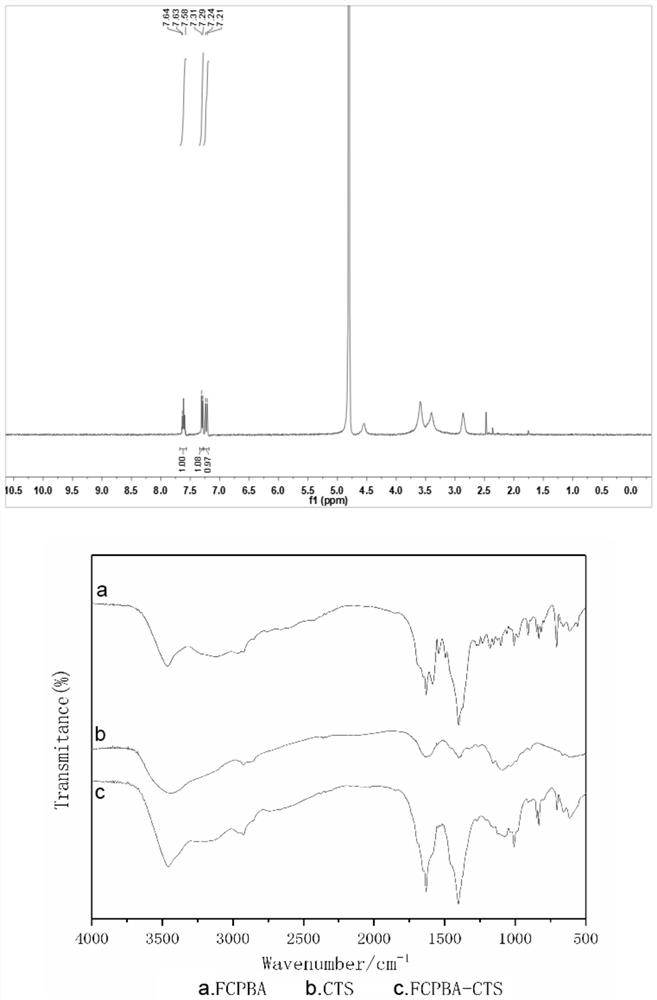
[0072] Example 3 Preparation of 4-carboxy-3-fluorophenylboronic acid grafted chitosan glucose-sensitive material
[0073] sample 1
[0074] Weigh 9.6mg EDC and 17.3mg NHS in the dark, fully dissolve in 1.5mL distilled water, add 2mg of 4-carboxy-3-fluorophenylboronic acid after 0.5h, add 8mg chitosan after fully dissolved, and wash with 0.01M hydrochloric acid Adjust the pH value to 4.7, stir and react at 4°C for 72 hours, concentrate with ultrafiltration centrifuge tube 5000rpm for 5min, freeze-dry the polymer of the gained 4-carboxy-3-fluorophenylboronic acid grafted chitosan, and in Store at 2-8°C until use (FCPBA-CTS).
[0075] sample 2
[0076] Weigh 9.6mg of EDC and 17.3mg of NHS in the dark, fully dissolve in 1.5mL of distilled water, add 4mg of 4-carboxy-3-fluorophenylboronic acid after 0.5h, add 8mg of chitosan after fully dissolved, and wash with 0.01M hydrochloric acid Adjust the pH value to 6, stir and react at 8°C for 24 hours, concentrate with ultrafiltration ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com