Application of RNA and carrier in preparation of product for preventing and/or treating liver cancer
A carrier and product technology, applied in the application field of RNA and carrier in the preparation of prevention and/or treatment of liver cancer products, can solve the problem of no drug treatment, etc., and achieve the effect of low human pathogenicity and wide host range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1, construction of miR-122, recombinant adenovirus vector containing miR-122 encoding gene and adenovirus expressing miR-122
[0057] 1. Acquisition of miR-122
[0058] Synthetic miR-122 was synthesized in Guangzhou Ruibo Biotechnology Co., Ltd.
[0059] The nucleotide sequence of miR-122 is sequence 1 in the sequence listing.
[0060] The negative control RNA of miR-122 was purchased from Guangzhou Ruibo Biotechnology Co., Ltd. (for miRNA mimic Ncontrol#22 product information:
[0061] Basic information: common miRNA mimic Ncontrol#22 for humans, mice, and rats
[0062] Mature miRNA name: cel-miR-239b-5p
[0063] Mature miRNA number: MIMAT0000295
[0064] Mature miRNA sequence: UUUGUACUACACAAAAGUACUG)
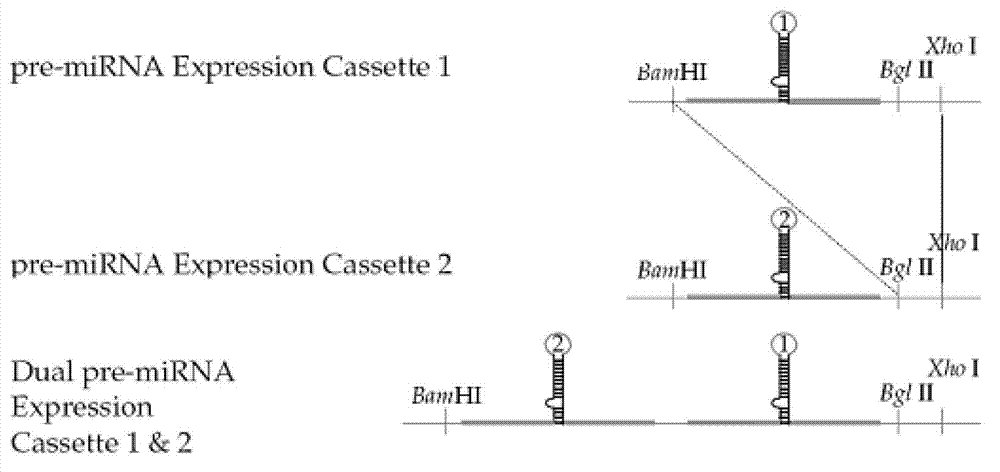
[0065] 2. Construction of adenovirus recombinant vector containing miR-122
[0066] The nucleotide sequence of the gene encoding the miR-122 precursor is sequence 3 in the sequence table, the gene encodes the miR-122 precursor, and miR-122 (sequence 1) is ...
Embodiment 2
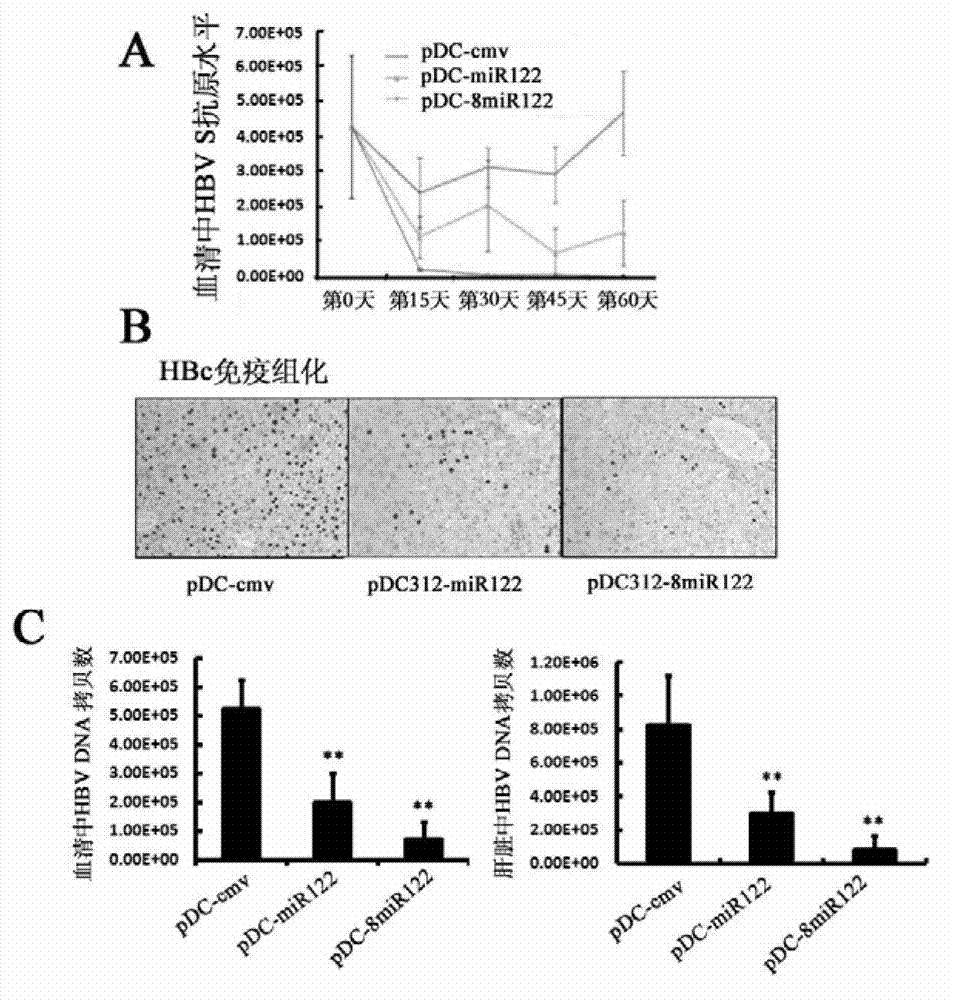
[0117] Example 2, application of miR-122, recombinant adenovirus vector containing miR-122 encoding gene and adenovirus expressing miR-122
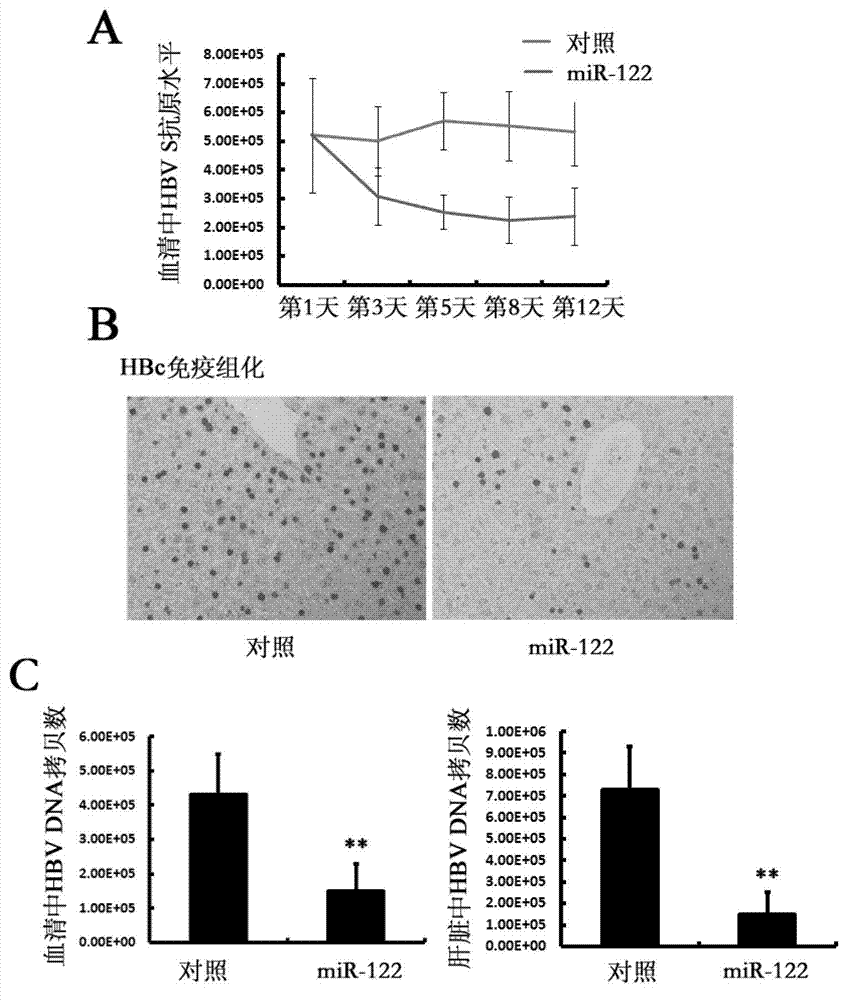
[0118] 1. Application of miR-122 and adenovirus expressing miR-122 in the treatment of HBV transgenic mice
[0119] A. The application of miR-122 in the treatment of HBV transgenic mice
[0120] HBV transgenic BALB / c mice aged 6-8 weeks (purchased from the Liver Disease Center of the 458th Hospital of the Chinese People's Liberation Army); mice with similar body weight, about 16g, were randomly divided into 2 groups, 5 mice in each group .
[0121] miR-122 solution: every 10nM miR-122 was dissolved in 0.1ml of PBS (8g / L NaCl, 0.2g / L KCl, 3.628g / LNa 2 HPO 4 12H 2 0.0.24g / L KH 2 PO 4 and water, pH 7.4), to obtain miR-122 solution;
[0122] Negative control RNA solution of miR-122: every 10nM of miR-122 negative control RNA was dissolved in 0.1ml of PBS (8g / LNaCl, 0.2g / L KCl, 3.628g / L NaCl 2 HPO 4 12H 2 0.0.24g / L KH 2 PO 4 and wa...
Embodiment 3
[0222] Example 3, miR-122, the vector containing the gene encoding miR-122 or the application of the adenoviral vector containing the gene encoding miR-122 in inhibiting HBV replication ability in vitro
[0223] 1. Application of miR-122 in inhibiting HBV replication ability
[0224] HepG2 cells were inoculated into 6-well plates one day before transfection, and the confluence of cells reached about 70% during transfection, and the medium was replaced with opti-MEM (life technologies catalog number: 31985070) one hour in advance.
[0225] miR-122 group: HepG2 cells were co-transfected with miR-122 and plasmid pHBV1.3 using lipo2000 to obtain HepG2 cells / miR-122; the optimal transfection ratio of RNA and pHBV1.3 replicating plasmid was 2.5 in volume and mass ul: 1ug, 4-6 hours the cells were washed three times with PBS, and replaced with 2ml of 10% FBS DMEM full medium.
[0226] Negative control group: the same method was used to co-transfect HepG2 cells with miR-122 negative ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com