Anti-PD-L1 antibody as well as application, preparation method, kit and drug
A PD-L1 and antibody technology, applied in biochemical equipment and methods, antibodies, anti-tumor drugs, etc., can solve the problems of difficult gene detection, inability to detect silence or expression, increase workload, etc., and achieve high specificity and affinity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] In another aspect, the present invention provides a method for preparing the above-mentioned anti-PD-L1 antibody, which comprises: immunizing animals with an antigenic peptide having an amino acid sequence as shown in positions 29-290 of SEQ ID No.2.
[0044] The present invention shows that the 29-290 position of PD-L1 has good antigenicity and contains B cell epitope through bioinformatics analysis, and the 29-290 region of PD-L1 is used as the recombinant protein (antigenic peptide) to immunize mice to prepare mouse monoclonal Anti-PD-L1 antibody, the obtained anti-PD-L1 antibody can recognize PD-L1 and has better specificity.
[0045] Of course, it should be noted that other animals such as rabbits, rats, pigs and other mammals can also be selected for immunization to prepare antibodies.
[0046] Further, in some embodiments of the present invention, before immunization, the preparation method further includes: transforming E. coli with an expression vector containi...
Embodiment 1
[0055] Construction of PD-L1 recombinant protein expression plasmid
[0056] According to the nucleic acid sequence in Genebank (GI: 20070268) and the encoded protein sequence of Q9NZQ7, the PD-L1 monoclonal is synthesized by gene synthesis corresponding to the amino acid sequence from the 29th to the 290th position of the PD-L1 protein (SEQ ID NO.2). The DNA sequence ((SEQ ID NO.1) was introduced with restriction sites EcoR I and Xho I, cloned into the expression vector pET41a (Novagen Company), and sequenced and analyzed, and the sequenced correct clones were selected for protein expression and purification.
Embodiment 2
[0058] PD-L1 recombinant protein expression and purification
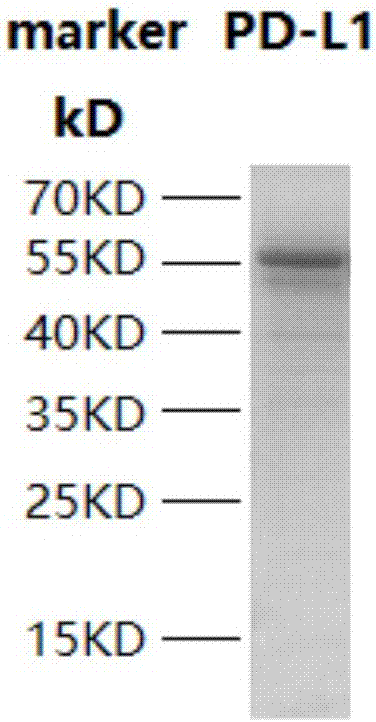
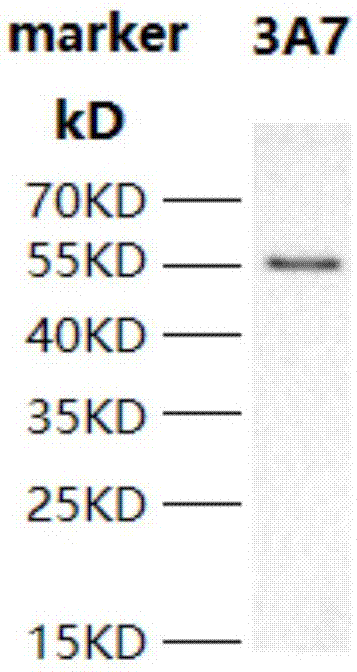
[0059] Escherichia coli containing the plasmid with the correct sequence of PD-L1 was cultivated to OD 600 0.5, add 10 μmol / L IPTG, culture overnight at 16°C, and after harvesting, sonicate and carry out Ni2 + Affinity purification. And carry out 12% SDS-PAGE detection, Coomassie brilliant blue staining, the result is as follows figure 1 As shown, the recombinant PD-L1 protein molecular weight is about 57kDa,
[0060] figure 1 The result (in the figure: marker is the molecular weight marker) shows that there is a band at the position of 55KD, indicating that the expression of PD-L1 recombinant protein obtained in this example is correct.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com