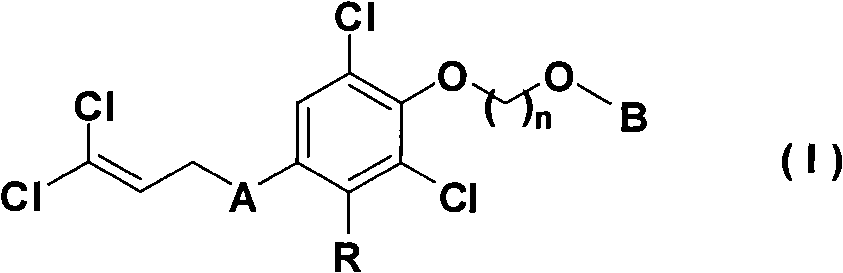
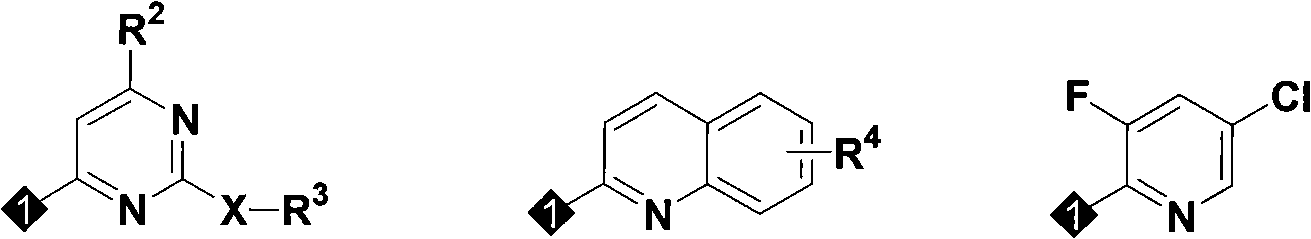
Nitrogen heterocyclic ring dichlorin allyl ether compounds with insecticidal activity
A technology of dichloroallyl ether and insecticidal activity, applied in the directions of biocides, chemicals for biological control, biocides, etc., to achieve the effect of high biological activity and good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] This example illustrates the preparation of compound 01 in Table 1.
[0090]
[0091] Step A: Synthesis of 2-methoxy-4-hydroxy-6-trifluoromethylpyrimidine (01-A)
[0092] Add 1.00 g of cyanamide into a 100 ml three-necked flask with a dry condenser, and add 5.00 g of HCl in methanol while stirring. Slowly raise the temperature to 25-80°C and react for 2-6 hours, cool in an ice-water bath to about 10°C, and then add 3.50 g of ethyl trifluoroacetoacetate dropwise. After the addition, adjust the pH value to 14 with 20% NaOH solution. Control the temperature below 30°C, raise the temperature to reflux after the addition, react for 5 to 6 hours, and desolvate under reduced pressure, add 20 grams of water to adjust the pH to be acidic, until the amount of precipitated solids is the largest, and dry to obtain about 3.0 g of the crude product. Content 96% (HPLC).
[0093] Step B: Synthesis of 2-methoxy-4-chloro-6-trifluoromethylpyrimidine (01-B)
[0094] Add 3.88g (20mmol...
Embodiment 2
[0106] This example illustrates the preparation of compound 06 in Table 1.
[0107]
[0108] Step A: Synthesis of 4-(3-bromopropoxy)-2-ethoxy-6-methylpyrimidine (06-A)
[0109] Synthesize with reference to the corresponding method in Example 1.
[0110] Step B: 4-(3-(2,6-dichloro-4-(3,3-dichloroallyloxy)phenoxy)propoxy-2-ethoxy-6-methylpyrimidine ( 06) Synthesis
[0111] In a 100ml three-necked flask equipped with a dry condenser, add 2.6g (06-A), 2.7g (01-F), 1.3g potassium carbonate and 20ml DMF, react at room temperature for 10-20 hours, pour into water, wash with water, and acetic acid Extracted with ethyl ester, dried over anhydrous sodium sulfate, and removed the solvent under reduced pressure to obtain 5.4 g of yellow liquid, content 42% (HPLC), yield 51%, through column chromatography (petroleum ether: ethyl acetate = 100:1) After purification, the title compound (06) was obtained as a yellow sticky solid, the structure of which was confirmed by mass spectrometry...
Embodiment 3
[0113] This example illustrates the preparation of compound 08 in Table 1.
[0114]
[0115] Step A: Synthesis of 4-(3-bromopropoxy)-2-methylthio-6-trifluoromethylpyrimidine (08-B)
[0116] In a 100ml three-neck flask equipped with a dry condenser, add 3.5g 2-methylthio-4-chloro-6-trifluoromethylpyrimidine, 2.75g 3-bromopropanol, 2.75g potassium carbonate and 20mlDMF, and react at room temperature for 8 hours, the reaction was completed, poured into water, washed with water, extracted with ethyl acetate, dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure to obtain 5.08 g of the title compound as a yellow liquid with a content of 86% (HPLC).
[0117] Step B: 4-(3-(2,6-Dichloro-4-(3,3-dichloroallyloxy)phenoxy)propoxy-2-methylthio-6-(trifluoromethyl base) synthesis of pyrimidine (08)
[0118] Add 50ml of DMF, 3.3g 2-methylthio-4-(3-bromopropoxy)-6-trifluoromethylpyrimidine, 3.4g 4-(3 , 3-dichloropropenyloxy) phenol, 3.4g of anhydrous pot...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com