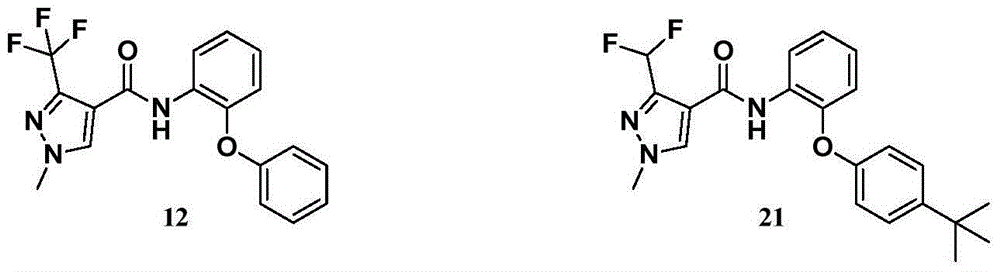Pyrazole amide compound containing diphenyl ether, and application and pesticide composition of pyrazole amide compound
A pesticide composition, pyrazole amide technology, applied in the field of pyrazole amide compounds, can solve the problems of poor control effect of diseases and insect pests, poor control effect of rice sheath blight and cucumber powdery mildew, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Synthesis of diphenyl ether-containing pyrazole amide compounds shown in formula I and formula II.
[0036] Refer to synthetic route (1) and synthetic route (2) as described above.
[0037] 1. Synthesis of 2,4-dichloro-1-(2-nitrophenoxy)benzene
[0038] 2.82g (20mmol) 2-fluoronitrobenzene and 4.89g (30mmol) 2,4-dichlorophenol, 4.15g (30mmol) K 2 CO 3 Add 50ml DMF and 50ml DMF to a 100ml single-necked bottle, heat to reflux for 5h, remove DMF under reduced pressure, wash three times with 10% NaOH aqueous solution, extract with ethyl acetate, anhydrous Na 2 SO 4 After drying, it was precipitated to obtain 5.50 g of 2,4-dichloro-1-(2-nitrophenoxy)benzene, with a yield of 97%.
[0039] 2. Synthesis of 2-(2,4-dichlorophenoxy)aniline
[0040] Dissolve 5.50g (19.36mmol) of 2,4-dichloro-1-(2-nitrophenoxy)benzene in 100ml of ethanol, add 1.04g (19.36mmol) of ammonium chloride and 10ml of water, heat to reflux and add Three batches of 3.24g (58.08mmol) iron powder were reac...
preparation example 1
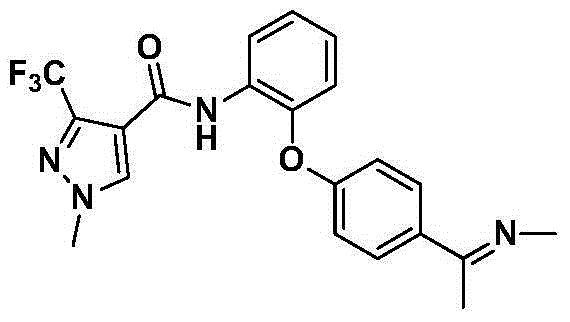
[0051] Compounds shown in the following formula (III) that are structurally similar to the pyrazole amide compounds shown in formula I and / or formula II were synthesized through a synthetic route similar to that of Example 1, and their numbers are shown in Table 1.
[0052]
[0053] Table 1
[0054] serial number R 6 R 2 R 3 R 4 R 5 III-1 CF 3 H Cl H H III-2 CF 3 Cl H Cl H III-3 CF 3 H H F H III-4 CF 3 H Ome Ome Ome III-5 CHF 2 H Ome Ome Ome III-6 CF 3 H H CN H III-7 CHF 2 H H CN H III-8 CF 3 Cl H H H III-9 CF 3 H H Cl H III-10 CHF 2 H H Cl H III-11 CF 3 H H CF 3 H III-12 CHF 2 H H CF 3 H III-13 CF 3 CH 3 CH 3 H H III-14 CHF 2 CH 3 CH 3 H H III-15 CF 3 H CH 3 H CH 3 III-16 CHF 2 H CH 3 H CH 3
[0055] The relevant nuclear magnetic data ...
preparation example 2
[0073] According to the methods disclosed in Examples 12 and 21 of CN 1226244A, compound 5, compound 12 and compound 21 shown in the following formula were prepared.
[0074]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com