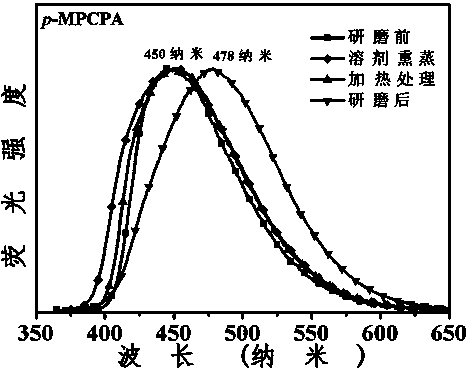
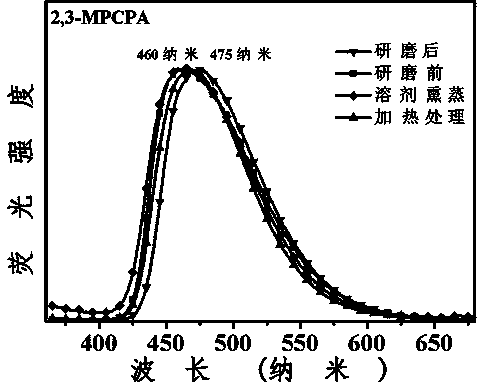
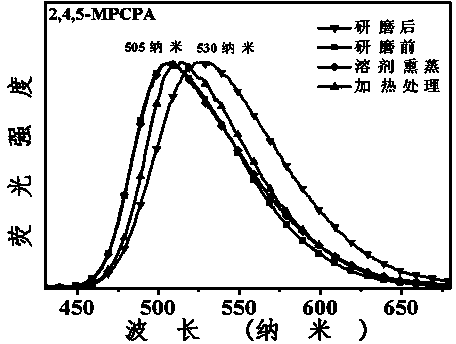
3-aryl-2-cyanoacrylamide derivative as well as preparation method and application thereof
A technology of cyanoacrylamide and derivatives, applied in the application of pressure-sensitive materials, organic small molecules and its preparation field, can solve the problems of lack of in-depth understanding of the formation mechanism of discoloration phenomenon, rare types of materials, limited quantities, etc., and achieve good results Reversible stimulus-responsive mechanochromic properties, low equipment requirements, and convenient post-processing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Preparation of 2-cyano-3-(4-methoxyphenyl)-2-acrylamide
[0038] Add 4-methoxybenzaldehyde (1.36g, 10mmol), cyanoacetamide (0.84g, 10mmol), L-proline (0.058g, 0.5mmol) into 15ml of absolute ethanol in sequence, stir and heat to reflux After the reaction of 4-methoxybenzaldehyde is complete (TLC tracking reaction), cool to room temperature, a white solid precipitates, vacuum filter, wash the filter cake with a mixed solution of ethanol and water (volume ratio 1:1), and dry 1.82 g of white solid product was obtained with a yield of 90.1%.
[0039] 1 H NMR (500 MHz, DMSO): δ 8.11 (s, 1H), 7.97 (d, J = 8.8 Hz, 2H), 7.79 (s, 1H), 7.73 – 7.59 (m, 1H), 7.14 (t, J = 5.8 Hz, 2H), 3.86 (s, 3H). 13 C NMR (126 MHz, DMSO): δ 163.1, 162.6, 150.1, 132.4, 124.4, 117.0, 114.8, 102.9, 55.6, 54.9.
[0040] In the above examples, 4-methoxybenzaldehyde is replaced by other methoxy-substituted benzaldehydes, all of which can achieve the same technical effect.
Embodiment 2
[0042] With the operation of Example 1, the solvent is anhydrous methanol, and the 4-methoxybenzaldehyde: cyanoacetamide: the amount of substance ratio of L-proline is changed from 1:1:0.05 to 1:1:0.10, 1.85 g of white pure product was obtained with a yield of 91.6%.
Embodiment 3
[0044]With the operation of Example 1, the amount ratio of 4-methoxybenzaldehyde: cyanoacetamide: L-proline is changed from 1:1:0.05 to 1:1:0.15 to obtain 1.87g of white pure product , and the yield was 92.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com