A process for the preparation of entacapone form-A
A technology of cyano group and dihydroxy group, applied in the field of A-type crystallization, can solve the problems such as the type of polytype is not disclosed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
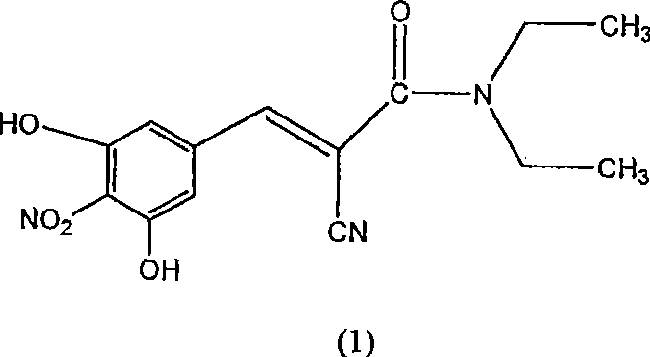
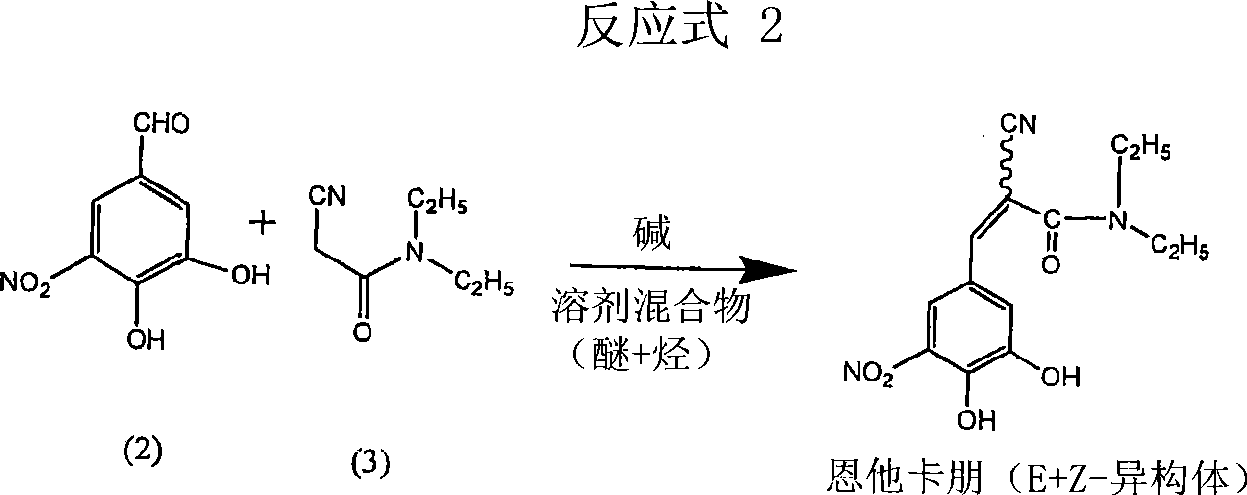
[0030] Preparation of (2E)-2-cyano-3-(3,4-dihydroxy-5-nitrophenyl)-N,N-diethyl-2-acrylamide (entacapone).
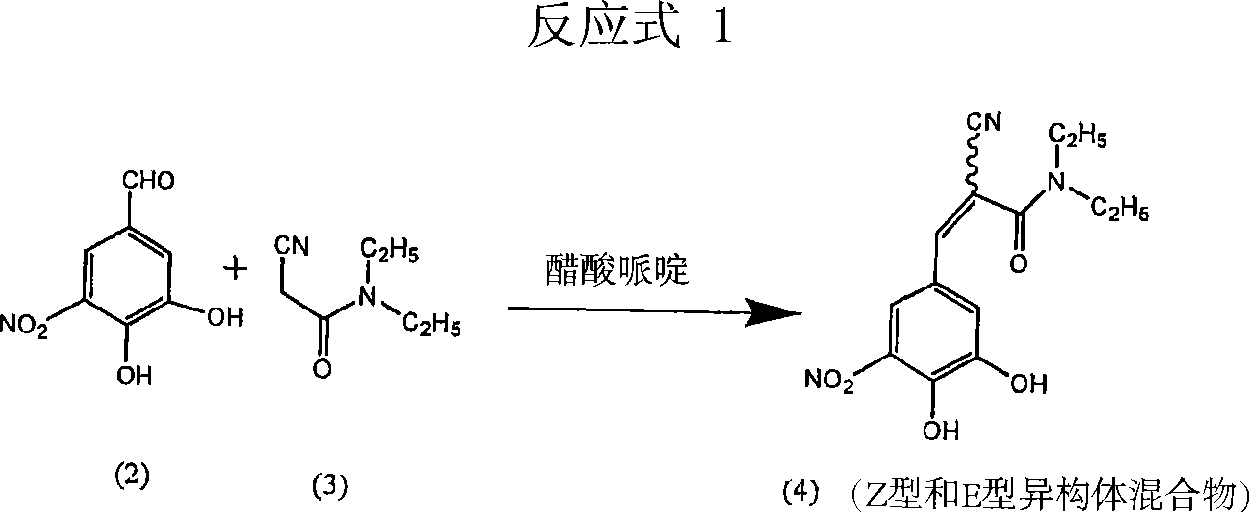
[0031]3,4-dihydroxy-5-nitrobenzaldehyde (50g), N,N-diethylcyanoacetamide (76.5g), piperidine (19.2g) were loaded into a mixture containing dimethoxyethane (200ml ) and heptane (200ml) in a reaction vessel. The reaction mixture was refluxed for 15-25 hours until the disappearance of starting material (monitored by HPLC). The solvent was distilled off under high vacuum at 70-80°C. Then cool to 25-35°C. To the intermediate product in the flask was added dichloromethane (1.0 L) at 25-35°C. The mixture was stirred at this temperature for 24 hours. It was then filtered, dried and charged to a reaction flask containing methanol. The mixture was treated with activated carbon, filtered and concentrated to obtain the target compound (HPLC 99.78%, Z-isomer content <0.1%) with a yield of 75%.
Embodiment 2
[0033] 3,4-dihydroxy-5-nitrobenzaldehyde (25g), N,N-diethyl cyanoacetamide (38.25g), dibutylamine (10g) were charged into dimethoxyethane (100ml ) and heptane (100ml) in a reaction vessel. The reaction mixture was refluxed for 15-25 hours until the disappearance of starting material (monitored by HPLC). Under high vacuum at 70-80°C, the solvent was distilled off. Then cool to 25-35°C. To the intermediate product in the flask was added dichloromethane (500ml) at 25-35°C. The mixture was stirred at this temperature for 24 hours. Then, it was filtered, washed with water, dried and charged to a reaction flask containing acetonitrile. The mixture was treated with charcoal, filtered and concentrated to give the title compound (HPLC 99.7%, Z-isomer content <0.1%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com