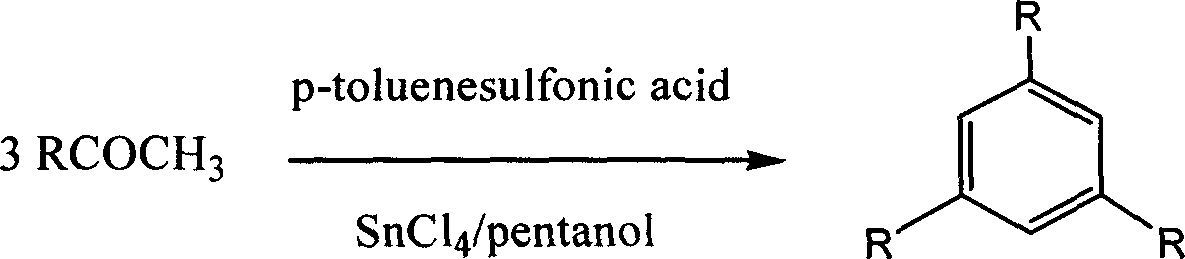Process for synthesizing1,3,5-triphenyl benzene
A triphenylbenzene, a new synthesis technology, applied in 1 field, can solve the problems of waste of energy and high-purity nitrogen, long reaction time, reduced yield and the like, and achieve the effects of easy recovery, easy operation, and reduced production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0018] Reaction formula of the present invention is:
[0019]
[0020] where R=(a)C 6 h 5 ; (b) 4-MeC 6 h 4 ; (c) 4-MeOC 6 h 4 ; (d) 4-ClC 6 h 4 ; (e) 4-BrC 6 h 4 ; (f)-Me; (g)4-NO 2 C 6 h 4
[0021] Specific steps:
[0022] Dissolve 1.2g of acetophenone in 10ml of dry anhydrous n-amyl alcohol, to prevent the water from destroying the tin tetrachloride added later and make the catalyst lose its effect, slowly drop in 5% mol of the catalyst tin tetrachloride under stirring, and then Add 5% mol of catalyst p-toluenesulfonic acid to form a composite catalyst. The amount is enough to ensure the normal progress of the reaction. React for 5 hours at a temperature of 110°C. After the reaction is completed, pour the reactant into 20ml of absolute ethanol , because the solubility of the product triphenylbenzene compound in ethanol is relatively small, so the product will be separated out in a large amount, then the ethanol solution is put into the refrigerator and cool...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com