Method of selective recovery of valuable metals from mixed metal oxides
A technology of mixtures and organic halides, applied in the direction of process efficiency improvement, photographic process, electrodes, etc., can solve the problems of high material cost and large amount of waste, achieve the effect of reducing cost and energy consumption requirements, and reducing environmental impact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 - Composition of bath formulations for recovery of indium / tin
[0026] The first bath formulation for the recovery of indium from pretreated ITO-containing particles mainly consists of the following two components:
[0027] a) One or more than one type of organic halide salts: The cations of these organic halide salts can be, but are not limited to, tetraalkylammonium, (dialkyl, trialkyl and tetraalkyl)imidazolium, alkane Dialkylpyridinium, dialkylpyrrolidinium, dialkylpiperidinium, tetraalkylphosphonium, tetraalkylsulfonium, dialkylpyrazolium and N-alkylthiazolium. In this example, 2-hydroxy-N,N,N-trimethylethylammonium chloride (choline chloride) was used as the organic halide salt in the first bath formulation;
[0028] b) 20-80 mol% dicarboxylic acid: The dicarboxylic acid may be, but not limited to, oxalic acid, malonic acid, succinic acid, glutaric acid and adipic acid.
[0029] In one embodiment, the molar ratio of the organohalide salt to the dicarbo...
Embodiment 2
[0034] Example 2 - Electrodeposition Conditions for Indium / Tin Collection from Bath Preparations Containing Dissolved Indium and / or Tin
[0035] In Example 1, after dissolving the pretreated particles of ITO-containing waste into the first bath formulation and adding 50-300% by volume of water, the solution was filtered and the indium-rich filtrate was separated from the tin-rich filter residue. To collect indium from said indium-rich filtrate, placing an indium plate into said filtrate to remove any residual tin in said filtrate, followed by a first electrodeposition process under the following conditions: pH not higher than 1.5; The current density is 0.6mA / cm 2 to 4mA / cm 2 Or the voltage is 2V to 4V, more preferably the voltage is about 2.6V; the electrodeposition time: 30-90 minutes; the temperature is 60°C to 120°C. Indium metal was electrochemically deposited on the substrate from the tin-rich filtrate in an electrochemical cell. The substrate can be, but is not limi...
Embodiment 3
[0037] Example 3 - Recovery of Indium and Tin from ITO-Containing Scrap Using the Bath Formulation and Electrodeposition Process of the Invention
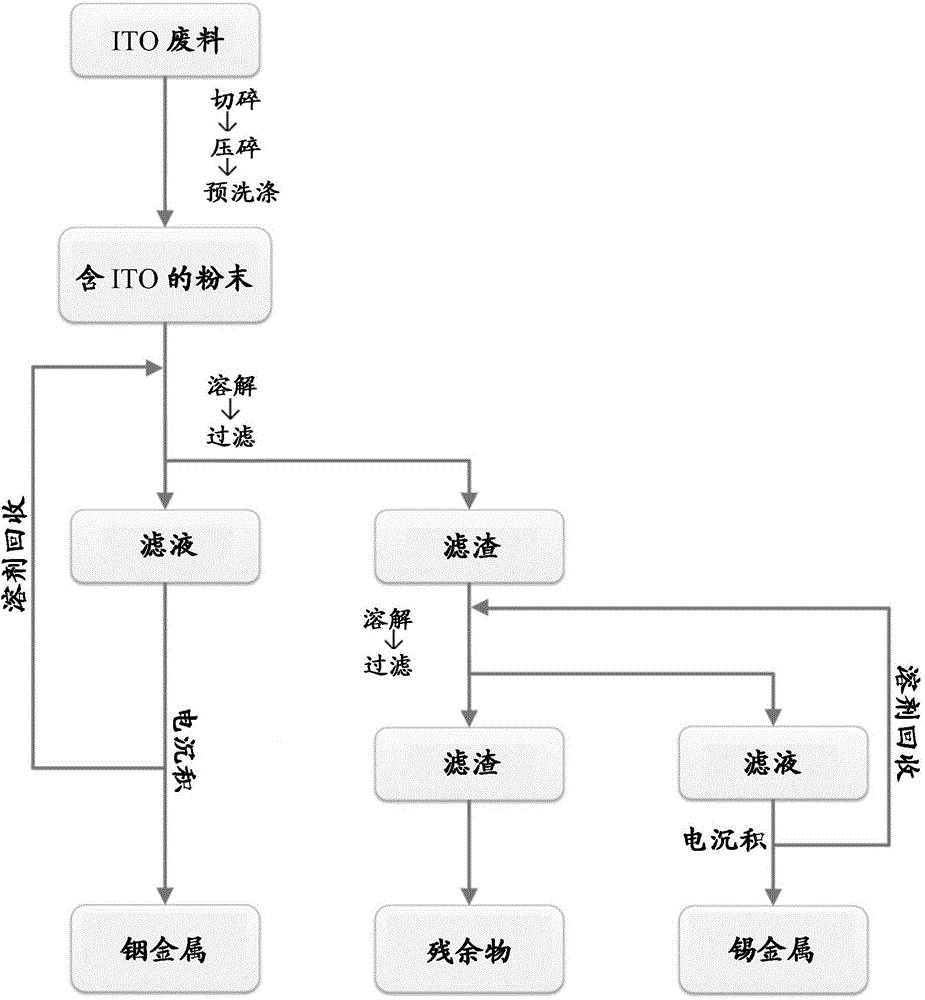
[0038] exist figure 1Among them, the recovery pathway of indium and tin consists of 3 main steps. First, the collected and produced ITO-containing waste is crushed to reduce the waste size, and chemically washed to eliminate those organic residues such as liquid crystals (LC) in liquid crystal displays (LCDs). Subsequently, the pretreated powder is transferred to a dissolution bath containing the corresponding ionic solvent, such as the first bath formulation in Example 1 for dissolving indium. In the first dissolution bath, indium and tin are dissolved out of the ITO (Equations 1 and 2) and are separated as In(X) 2 - and Sn(X) 2 The form of is stable (Equations 3 and 4).
[0039] In 2 o 3 +6H + =2In 3+ +3H 2 O………(Equation 1)
[0040] SnO 2 +4H + =Sn 4+ +2H 2 O…………..(Equation 2)
[0041] In 3+ +2X 2- =In(X) 2 - ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com