Method for preparing malononitrile oxime ether compound and intermediate compound
A technology of ether compounds and malononitrile oxime, which is applied in the field of preparation of malononitrile oxime ether compounds, methods and intermediate compounds, can solve the problems of high preparation cost and low product yield, and achieve reduction of process cost and yield High efficiency and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] The preparation method includes:
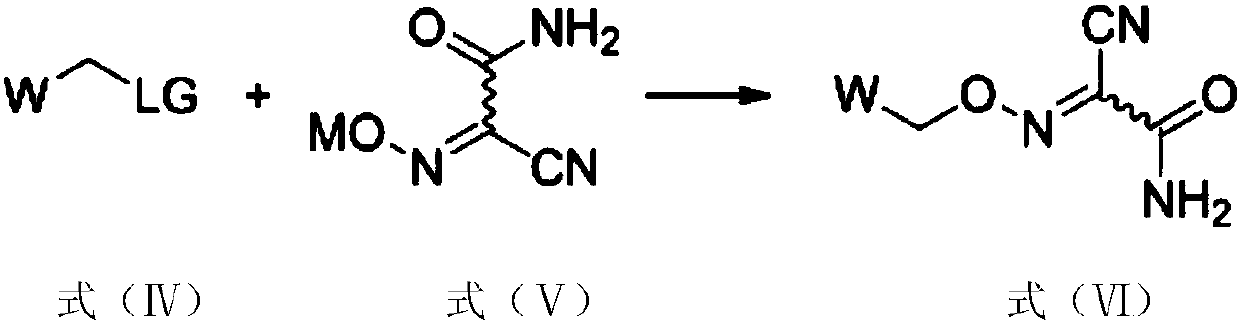
[0022] Under the action of the first solvent and the catalyst, the first raw material and the second raw material are reacted to obtain an intermediate compound, the first raw material has a structure shown in formula (IV), and the second raw material has a structure shown in formula (V), The synthetic route is as follows:
[0023]
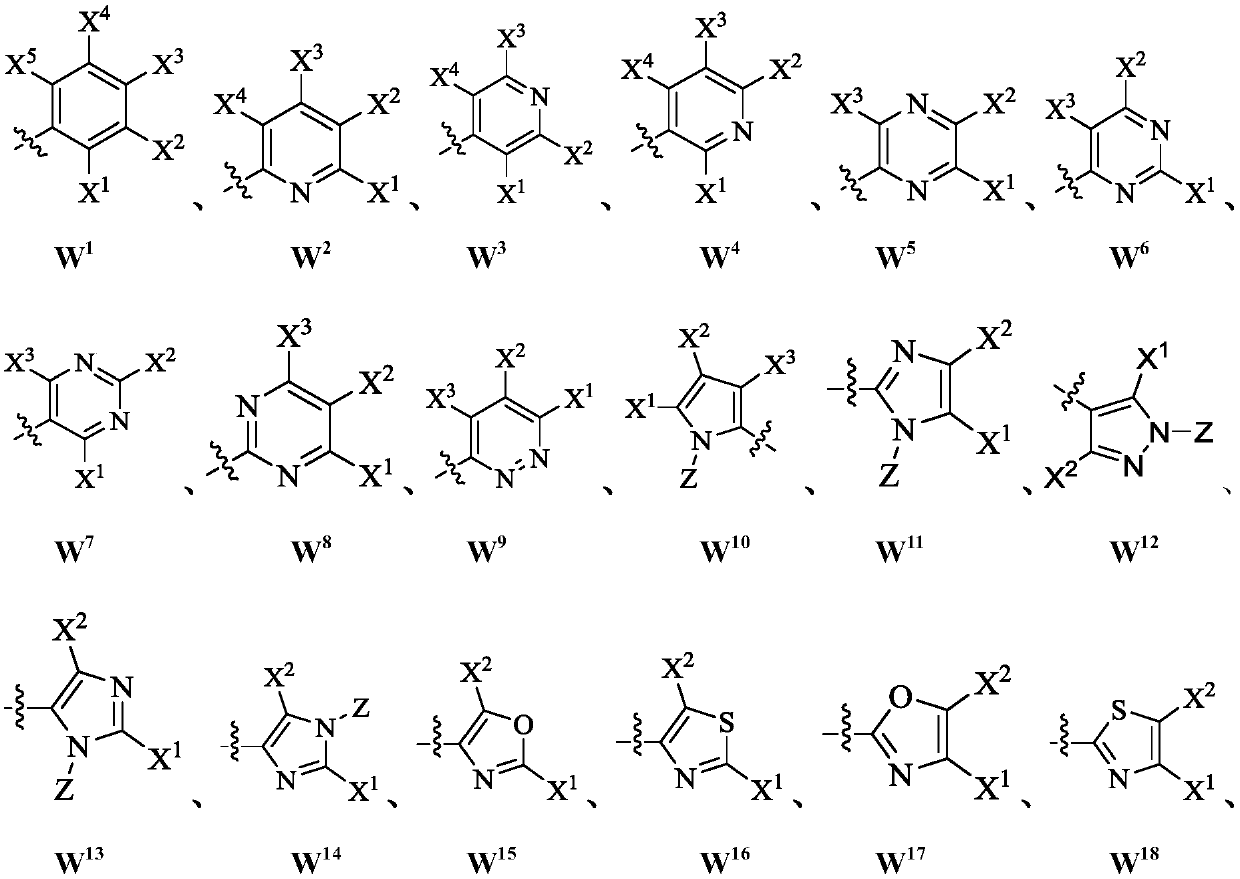
[0024] Wherein, LG is a leaving group; M is selected from monovalent cations; W is selected from aryl or heteroaryl; Represents a chemical bond, and the configuration of the double bond can be cis or trans;
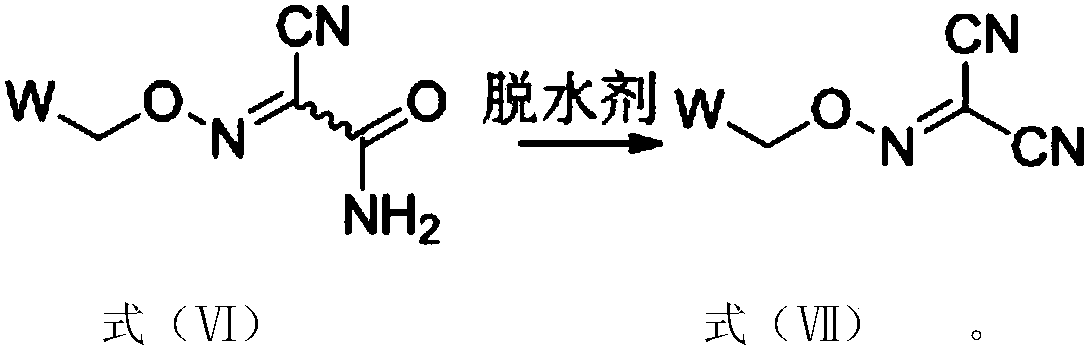
[0025] Under the effect of the second solvent, the intermediate compound shown in the formula (VI) is dehydrated with the dehydrating agent to obtain the malononitrile oxime ether compound, and the synthetic route is as follows:
[0026]
[0027] In the above-mentioned preparation method, the second raw material having the structure shown in formula (V) is prepared as raw material with cheap ...
Embodiment 1
[0136] 1) Preparation of 2-cyano-2-[((3,4-dichlorophenyl)methoxy)imino]acetamide (compound 35):
[0137]
[0138] Add 3,4-dichlorobenzyl chloride (9.87g, 50.0mmol), 2-cyano-2-hydroxyiminoacetamide sodium salt (10.66g, 75.0mmol), potassium iodide (0.84g, 5.0mmol) into the reaction flask mmol) and acetonitrile (80 mL), the temperature was raised to 80° C. for 10 h, and the reaction was monitored by TLC until the reaction was complete. Cool the reaction solution to room temperature, add ethyl acetate (100mL) and water (50mL) to dissolve, extract, stand to separate layers, wash the organic phase with saturated brine, dry over anhydrous magnesium sulfate, filter, and distill off the ethyl acetate under reduced pressure , the residue was separated and purified by column chromatography to obtain compound 35 (from the H NMR spectrum, isomer Z:E=1:1), white solid 11.00g, yield 80% (based on 3,4-dichlorobenzyl chloride ).
[0139] Compound 35 1 HNMR (600MHz, DMSO-d 6 )δ8.08(s,1H)...
Embodiment 2
[0145] 1) Preparation of 2-cyano-2-[((2-phenylthiazol-4-yl)methoxy)imino]acetamide (compound 60):
[0146]
[0147] Add 4-(chloromethyl)-2-phenylthiazole (10.59g, 50.0mmol), 2-cyano-2-hydroxyiminoacetamide sodium salt (11.37g, 80.0mmol), four Butylammonium bromide (0.82g, 2.5mmol) and acetone (80mL) were heated up to 60°C and kept for 12h. The reaction was monitored by TLC until the reaction was complete. Cool the reaction solution to room temperature, add ethyl acetate (100mL) and water (50mL) to dissolve, extract, stand to separate layers, wash the organic phase with saturated brine, dry over anhydrous magnesium sulfate, filter, and distill off the ethyl acetate under reduced pressure , the residue was separated and purified by column chromatography to obtain compound 60 (from the H NMR spectrum, isomer Z:E=1:1), white solid 11.86g, yield 82% (with 4-(chloromethyl)-2 - phenylthiazole).
[0148] Compound 60 1 HNMR (600MHz, CDCl 3 )δ7.95-7.93 (m, 2H), 7.46-7.45 (m, 3H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com