Preparation methods of flonicamid and intermediate 4-(trifluoromethyl)nicotinic acid thereof
A technology of trifluoromethylnicotinic acid and flonicamid, which is applied in the field of flonicamid preparation, can solve the problems of huge production cost and high cost of raw materials, and achieve the effects of low equipment requirements, high total yield and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
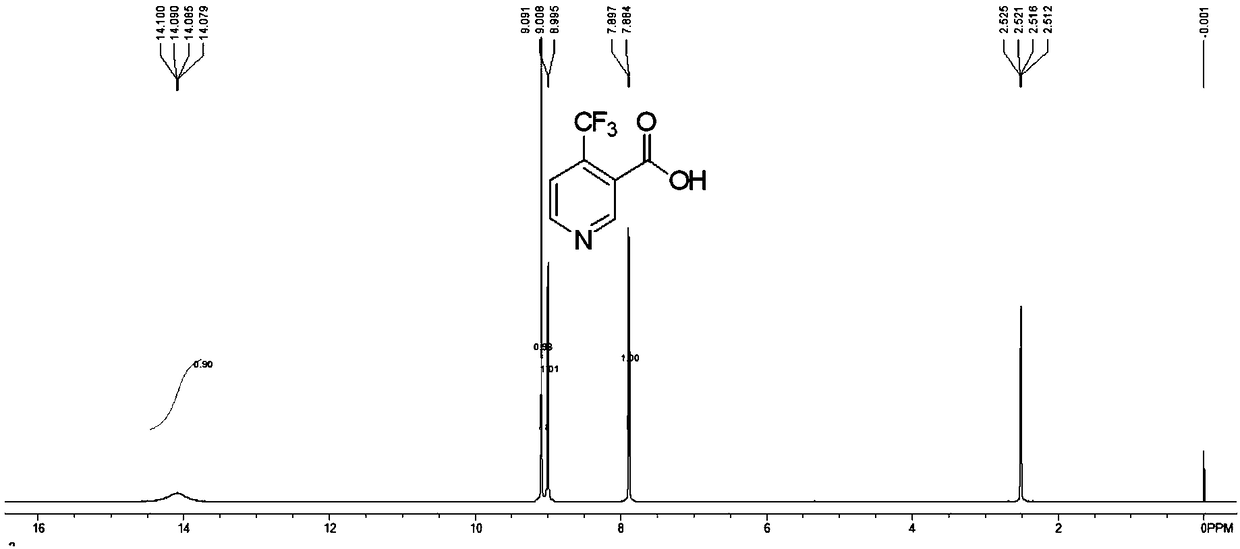
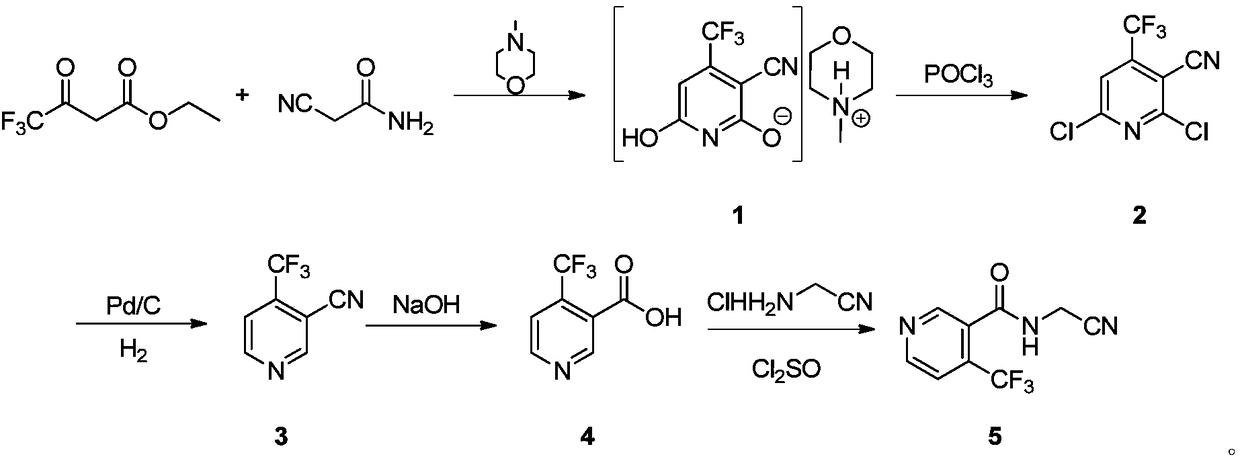
[0037] Add cyanoacetamide and ethyl trifluoroacetoacetate to N-methylmorpholine, reflux for 12 hours, collect the obtained solid with a Buchner funnel, and purify it with ethanol to obtain 2,6-dihydroxy -3-cyano-4-trifluoromethylpyridine N-methylmorpholine salt. Add phosphorus oxychloride to the obtained 2,6-dihydroxy-3-cyano-4-trifluoromethylpyridine N-methylmorpholine salt, reflux reaction for 18 hours, and recover excess oxygen trichloride by distillation under reduced pressure. After phosphorus, the raffinate was poured into a mixture of ice and water and extracted with ethyl acetate, and the organic phase was collected and concentrated to directly obtain 2,6-dichloro-3-cyano-4-trifluoromethylpyridine with high purity and high yield. Add the 2,6-dichloro-3-cyano-4-trifluoromethylpyridine obtained in the previous step into the solvent THF, add 10% Pd / C and triethylamine to carry out atmospheric pressure hydrogenation, and complete within 4 hours For the reaction, the solid...
Embodiment 2
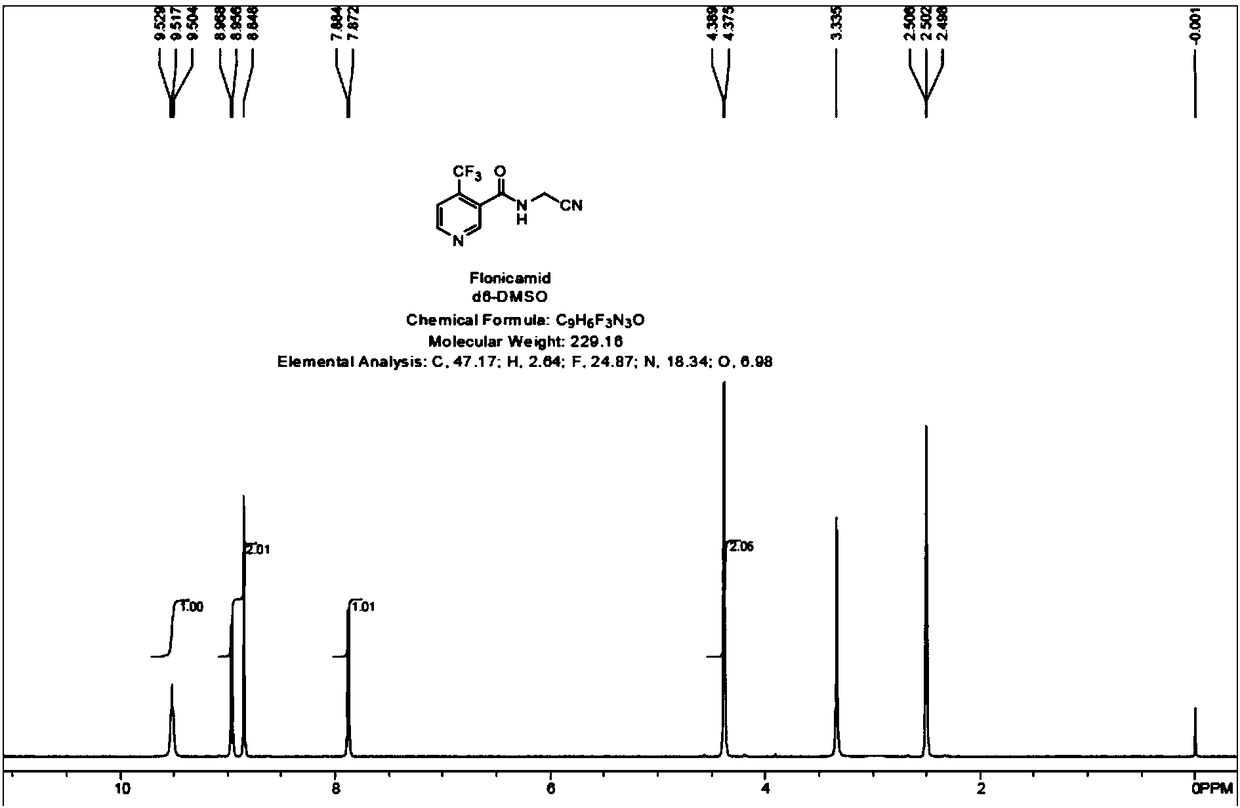
[0039] The preparation process of flonicamid is as follows:
[0040] 1) Weigh 10g (119mmol) of cyanoacetamide, add it to a 100mL three-necked reaction flask with a reflux condenser and a constant pressure dropping funnel, turn on the condensed water, and degas it with N 2 Protect. Add 30g (297mmol) N-methylmorpholine, stir well and add 20g (109mmol) ethyl trifluoroacetoacetate dropwise within 2h. Slowly raise the temperature to 78°C and keep the reaction for 12h. Afterwards, the reaction was cooled to -5°C, and the precipitated solid was filtered with a Buchner funnel, rinsed with 5g of ethanol, and the resulting product was dried at 70°C to obtain 27.6g (90.4mmol) of 2,6-dihydroxy-3-cyanide Base-4-trifluoromethylpyridinium N-methylmorpholine salt, yield 83.2%.
[0041] 2) Weigh 10g (32.8mmol) 2,6-dihydroxy-3-cyano-4-trifluoromethylpyridine N-methylmorpholine salt and 50g (326mmol) POCl 3 Add it to a 100mL three-neck reaction flask with a reflux condenser, and turn on the ...
Embodiment 3
[0046] The preparation process of flonicamid is as follows:
[0047] 1) Weigh 10g (119mmol) of cyanoacetamide, add it to a 100mL three-necked reaction flask with a reflux condenser and a constant pressure dropping funnel, turn on the condensed water, and degas it with N 2 Protect. Add 30g (297mmol) N-methylmorpholine, stir well and add 30g (163mmol) ethyl trifluoroacetoacetate dropwise within 2h. Slowly raise the temperature to 78°C and keep the reaction for 12h. Afterwards, the reaction was cooled to -5 °C, and the precipitated solid was filtered with a Buchner funnel, rinsed with 5 g of ethanol, and the resulting product was dried at 70 °C to obtain 33.2 g (109.1 mmol) of 2,6-dihydroxy-3-cyanide Base-4-trifluoromethylpyridinium N-methylmorpholine salt, yield 91.7%.
[0048] 2) Weigh 10g (32.8mmol) 2,6-dihydroxy-3-cyano-4-trifluoromethylpyridine N-methylmorpholine salt and 100g (326mmol) POCl 3 Add it to a 250mL three-neck reaction flask with a reflux condenser, and turn ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com