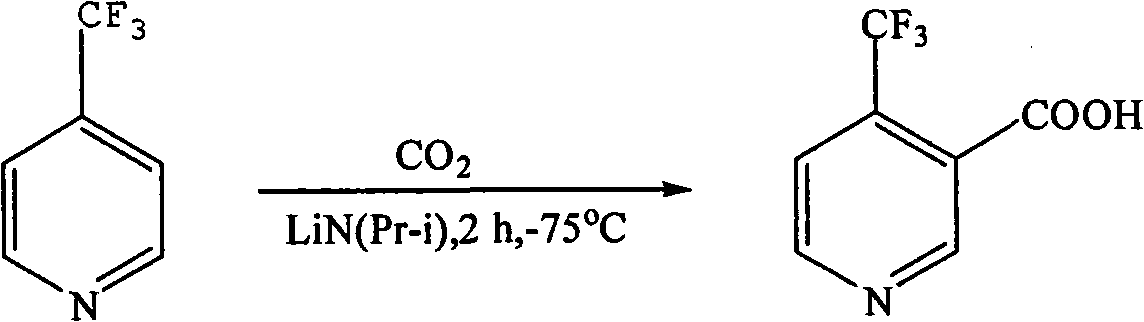Preparation method of 4-trifluoromethyl nicotinic acid
A technology of trifluoromethylnicotinic acid and trifluoromethylpyridine, which is applied in the field of compound preparation, can solve the problems of difficult realization, harsh conditions, and impossibility of industrialization, and achieve the effects of reduced production costs, fewer by-products, and low prices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
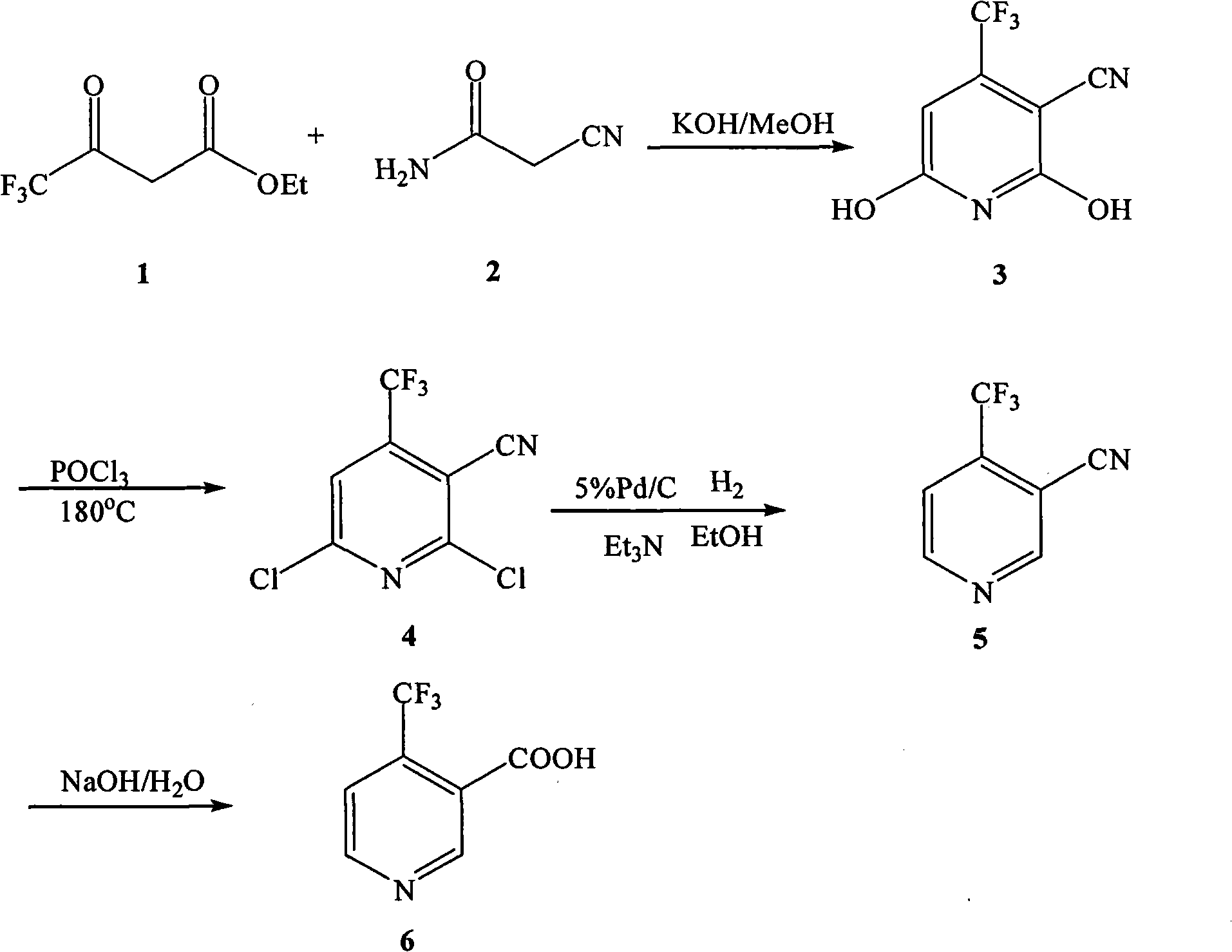
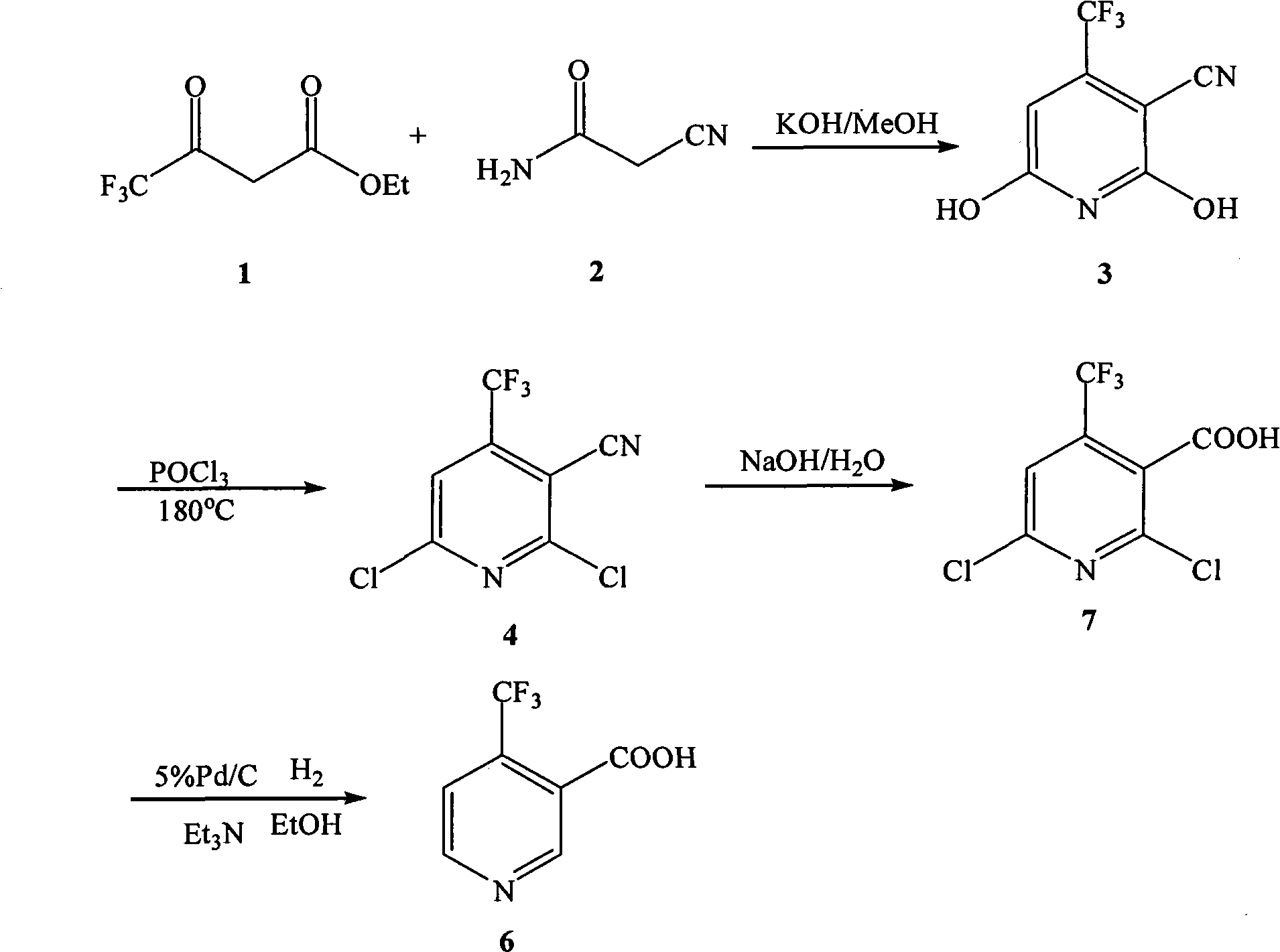
[0046] (1) Preparation of 2,6-dihydroxy-3-cyano-4-trifluoromethylpyridine
[0047]In a 100mL three-necked flask equipped with a reflux condenser and a thermometer, add 12g (0.142mol) of cyanoacetamide 2, 30mL of anhydrous methanol, and 20mL (0.136mol) of 4,4,4-trifluoroacetoacetic acid in sequence under stirring Ethyl ester 1. Magnetic force. Heat and stir in an oil bath until it reaches reflux. After the cyanoacetamide is completely dissolved, slowly add the prepared methanolic potassium hydroxide solution (9g / 20mL) dropwise, and drop it over within 24 hours. After dissolution, the color of the solution gradually deepened from yellow to orange-red, and then a white precipitate began to form. During the reaction, a small amount was dissolved in absolute ethanol for TLC detection, and the reflux continued for 6 hours. After the reaction was completed, it was left to stand, cooled, taken out for suction filtration, washed with methanol, water, and methanol in sequence, and the ...
Embodiment 2
[0055] (1) Preparation of 2,6-dihydroxy-3-cyano-4-trifluoromethylpyridine
[0056] In a 100mL three-necked flask equipped with a reflux condenser and a thermometer, add 12g (0.142mol) of cyanoacetamide 2, 30mL of anhydrous methanol, and 20mL (0.136mol) of 4,4,4-trifluoroacetoacetic acid in sequence under stirring Ethyl ester 1. Magnetic force. Heat and stir in an oil bath until it reaches reflux. After the cyanoacetamide is completely dissolved, slowly add the prepared methanolic potassium hydroxide solution (9g / 20mL) dropwise, and drop it over within 24 hours. After dissolution, the color of the solution gradually deepened from yellow to orange-red, and then a white precipitate began to form. During the reaction, a small amount was dissolved in absolute ethanol for TLC detection, and the reflux continued for 6 hours. After the reaction was completed, it was left to stand, cooled, taken out for suction filtration, washed with methanol, water, and methanol in sequence, and the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com