Preparation method and equipment of 4-trifluoromethyl-nicotinic acid
A technology of trifluoromethylnicotinic acid and trifluoromethylnicotinic acid amide, which is applied in the field of 4-trifluoromethylnicotinic acid preparation, can solve the problems of high cost and achieve the effects of low cost, reduced reduction rate and increased yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
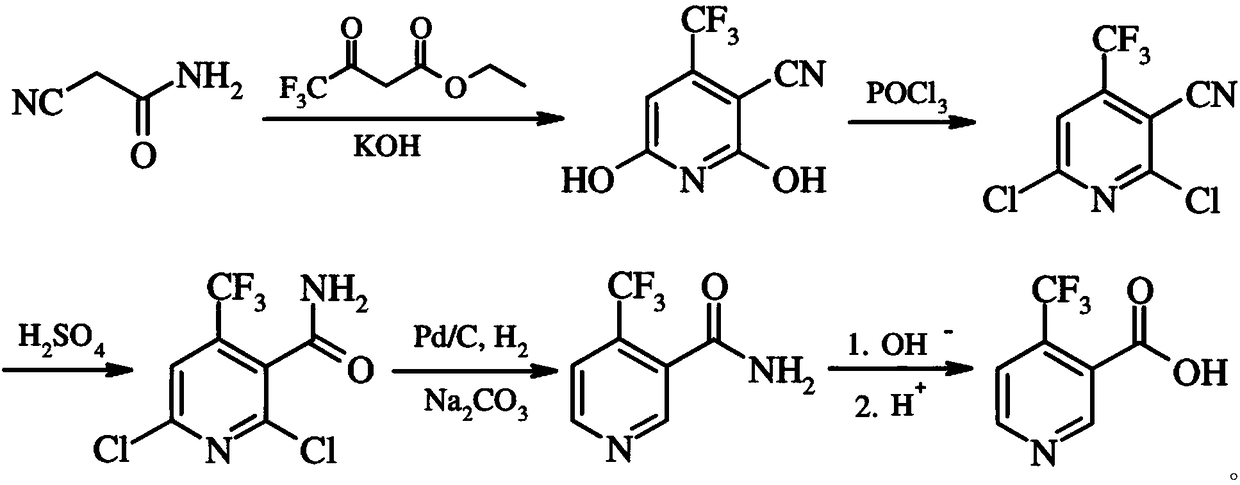
[0022] A preparation method of 4-trifluoromethyl nicotinic acid, comprising the following steps:
[0023] 1. After mixing cyanoacetamide, ethyl trifluoroacetoacetate and KOH, reflux at 75°C for 6 hours, then filter, wash and dry to obtain 2,6-dihydroxy-3-cyano-4 - trifluoromethylpyridine;
[0024] 2. Dissolve 2,6-dihydroxy-3-cyano-4-trifluoromethylpyridine in dichloroethane, then add phosphorus oxychloride and mix, then add dropwise N,N dimethylformamide, in Reflux at 125°C for 6 hours, cool, add to ice-water mixture, stir and separate layers, extract the water layer with dichloroethane, combine organic layers, dry over anhydrous sodium sulfate, filter with suction, and obtain 2,6- Dichloro-3-cyano-4-trifluoromethylpyridine;
[0025] 3. Mix 2,6-dichloro-3-cyano-4-trifluoromethylpyridine with sodium bromide, then add 80% sulfuric acid dropwise to the solution, and obtain 2,6-dichloro- 4-trifluoromethylnicotinamide;
[0026] 4. Add 2,6-dichloro-4-trifluoromethylnicotinamide ...
Embodiment 2
[0030] In step 4, feed nitrogen before high-pressure hydrogenation, carry out N 2 Replace 3-4 times to avoid leaving air in the autoclave during hydrogenation.
Embodiment 3
[0032] A preparation method of 4-trifluoromethyl nicotinic acid, comprising the following steps:
[0033] 1. After mixing cyanoacetamide, ethyl trifluoroacetoacetate and KOH, reflux at 80°C for 10 hours, then filter, wash and dry to obtain 2,6-dihydroxy-3-cyano-4 - trifluoromethylpyridine;
[0034] 2. Dissolve 2,6-dihydroxy-3-cyano-4-trifluoromethylpyridine in dichloroethane, then add phosphorus oxychloride and mix, then add dropwise N,N dimethylformamide, in Reflux at 135°C for 10 h, cool, add to ice-water mixture, stir and separate layers, extract the water layer with dichloroethane, combine organic layers, dry over anhydrous sodium sulfate, filter with suction, and obtain 2,6- Dichloro-3-cyano-4-trifluoromethylpyridine;
[0035] 3. Mix 2,6-dichloro-3-cyano-4-trifluoromethylpyridine with sodium bromide, then add 80% sulfuric acid dropwise to the solution, and obtain 2,6-dichloro- 4-trifluoromethylnicotinamide;
[0036] 4. Add 2,6-dichloro-4-trifluoromethylnicotinamide in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com