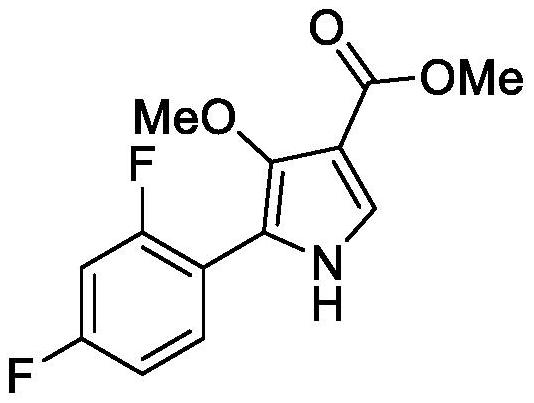
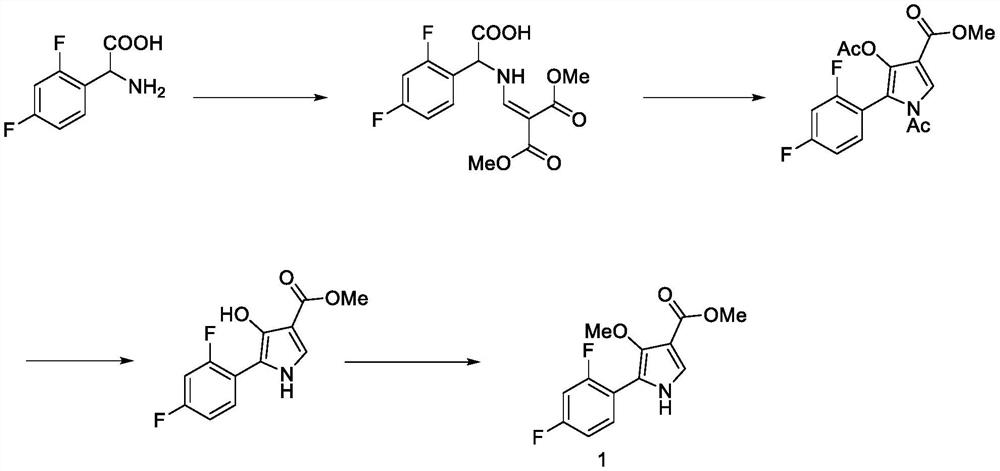
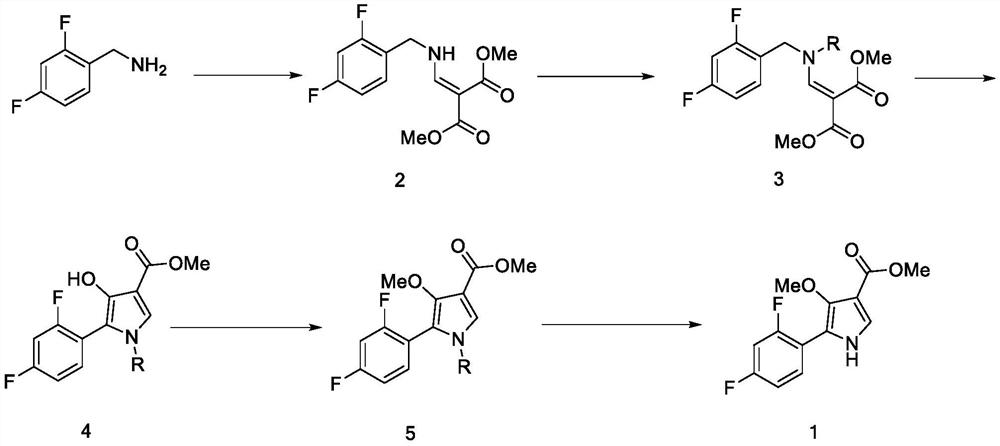
A kind of method for preparing potassium ion competitive blocker intermediate
A technology of reagents and condensing agents, applied in the field of organic chemical synthesis of pharmaceutical intermediates, can solve the problems of difficulty in meeting the needs of the pharmaceutical industry, expensive starting materials, low yields, etc., and achieves a novel process route, low cost, and mild reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043]
[0044] 1. Synthesis of compound 2
[0045] Add 66.7 g of dimethyl malonate and 60.1 g of N,N-dimethylformamide dimethyl acetal to a 500-ml reaction flask, warm up to 70 ° C for 2 hours, cool to room temperature, add 65 g of 2 , 4-difluorobenzylamine, the temperature was raised to 70°C and the reaction was continued for 2 hours. After cooling to room temperature, 500 ml of water and 500 ml of ethyl acetate were added, stirred for 10 minutes, left to separate layers, the organic phase was washed with saturated brine, dried with anhydrous sulfuric acid, concentrated under reduced pressure to remove the solvent, and the residue was added with n-heptane Slurried, filtered, and dried to give 111.1 g of compound 2, 85.8% yield, as a white solid.
[0046] 1 H-NMR (400MHz, d 6 -DMSO): 9.40(br,1H), 8.14-8.18-(m,1H), 7.42-7.49(m,1H), 7.27-7.32(m,1H), 7.12-7.17(m,1H), 4.64( d, 2H), 3.64(d, 3H), 3.60(d, 3H); ESI-MS: m / z 286.14[M+1]
[0047] 2. Synthesis of compound 3A
[...
Embodiment 2
[0060]
[0061] 1. Synthesis of compound 2
[0062] In a 500mL reaction flask, add 50.8 g of dimethyl malonate, 74.1 g of trimethyl orthoformate, and 71.3 g of acetic anhydride, heat to 100 °C for 2 hours, then drop to room temperature, add 50 g of 2,4-dimethy Fluorobenzylamine, the temperature was raised to 90°C and the reaction was continued for 2 hours. The temperature was lowered to room temperature, 500 ml of water and 500 ml of ethyl acetate were added, stirred for 10 minutes, and the layers were separated. The residue was added with n-heptane to be slurried, filtered, and dried to obtain 83.9 g of compound 2 with a yield of 84.2% and a white solid.
[0063] 2. Synthesis of compound 3B
[0064] 20 g of compound 2, 45.7 g of cesium carbonate powder, 200 ml of N,N-dimethylformamide, and 11.5 g of 4-methoxybenzyl chloride were added to a 500-ml reaction flask, and heated to 80 °C under nitrogen protection for the reaction. 2 hours. After cooling to room temperature, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com