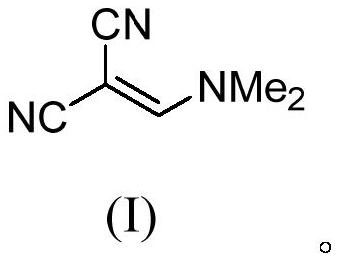
Method for continuously preparing (dimethylamine methylene)malononitrile by using micro-reaction system
A dimethylamine methylene and micro-reaction technology, which is applied in the field of continuous preparation of (dimethylamine methylene) malononitrile using a micro-reaction system, can solve the problems of high process cost, long reaction time, and high price. Achieve good separation effect, shorten reaction time and improve yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
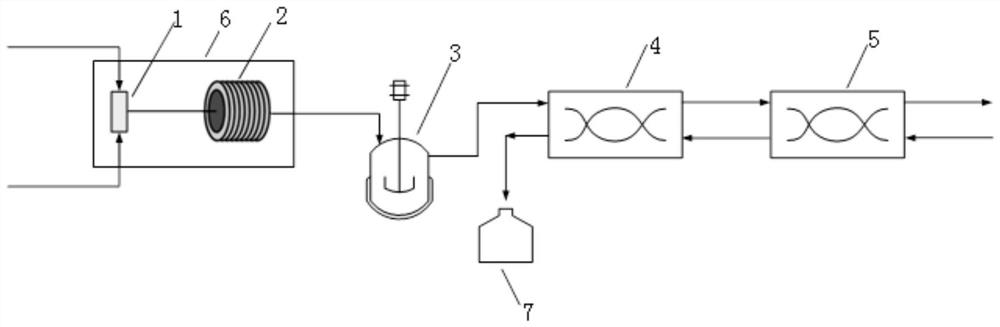
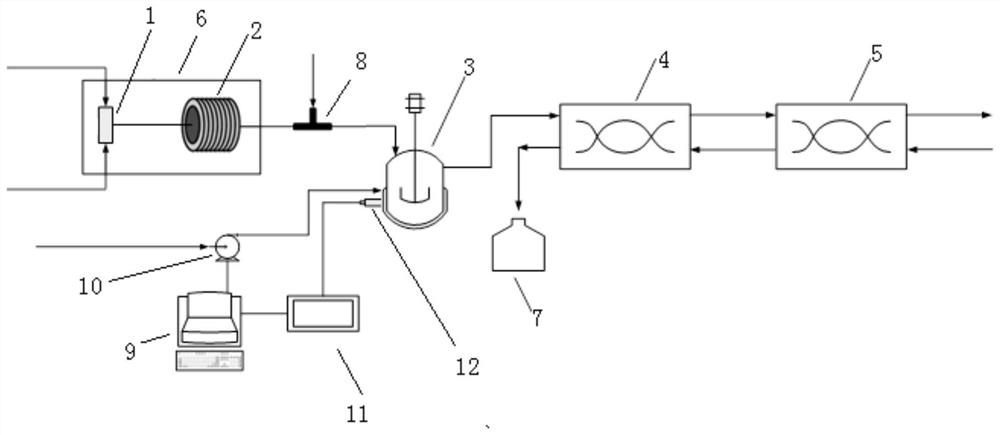
[0049] This embodiment provides a method for continuously preparing (dimethylaminomethylene) malononitrile using a micro-reaction system, the micro-reaction system, such as figure 1 As shown, it includes the first micro-mixer 1, microchannel reactor 2, pH adjustment tank 3, the first annular gap centrifugal extractor 4, and the second annular gap centrifugal extractor 5 connected in sequence, wherein the first annular gap Centrifugal extractor 4 and the second annular gap centrifugal extractor 5 are connected in series to form a centrifugal extraction unit, and the micro-reaction system also includes a constant temperature oil bath 6 and a storage tank 7, and the constant temperature oil bath 6 is used to adjust and control the microchannel The reaction temperature in Reactor 2. The first micro-mixer 1 is a T-shaped micro-mixer. The microchannel reactor 2 is a polytetrafluoroethylene tubular microchannel reactor with an inner diameter of 0.8 mm, and its reaction volume is 4 m...
Embodiment 2
[0055] This embodiment provides a method for continuously preparing (dimethylaminomethylene) malononitrile using a micro-reaction system. The micro-reaction system is the same as the micro-reaction system in Example 1, except that only one ring Gap centrifugal extractor.
[0056] The method comprises the steps of:
[0057] (1) The solution obtained after placing 0.06mol of cyanoacetamide, 0.18mol of N,N-dimethylformamide, and 0.006mol of pyridine in a dry reaction flask and mixed with 25.08g of phosphorus oxychloride They were simultaneously pumped into the first micro-mixer 1 for mixing to obtain mixed reaction materials, wherein, the solution obtained after mixing cyanoacetamide, N,N-dimethylformamide and pyridine (with a density of 1.105g / mL ) is pumped in at a flow rate of 0.232mL / min, and phosphorus oxychloride is pumped in at a flow rate of 0.212mL / min. The molar ratio is 1:3:0.1:2.726;
[0058] (2) The mixed reaction material flowing out from the first micro-mixer 1 ...
Embodiment 3
[0062] This embodiment provides a method for continuously preparing (dimethylaminomethylene) malononitrile using a micro-reaction system, the micro-reaction system is the same as the micro-reaction system of Example 1, and the method is the same as that of Example 1 , the difference is that the first micro-mixer 1 is a Y-type micro-mixer.
[0063] In this example, the reaction substrate cyanoacetamide was completely converted, and the yield of the product (dimethylaminomethylene) malononitrile was 96.1%, and the purity was 98.2% (GC).
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com