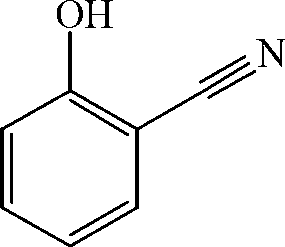
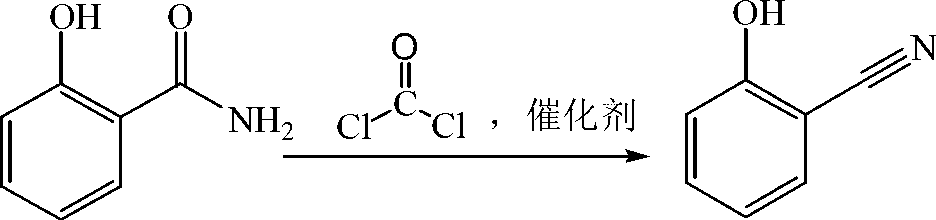
Preparation method of salicylonitrile
A technology of o-hydroxybenzonitrile and o-hydroxybenzamide, which is applied in the dehydration preparation of carboxylic acid amides, organic chemistry, etc., can solve problems such as being unsuitable for large-scale production, complicated process, difficult to remove, etc. Simple and safe, high-yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] 13.7g (0.1mol) o-hydroxybenzamide, 41.1g (0.39mol) xylene, 0.014g (1.6×10 -4 mol) dioxane was added to a 100mL three-necked flask with a stirring and reflux tube, heated to reflux, and 11.9g (0.12mol) phosgene was introduced within 2hr.
[0015] After 0.5hr of heat preservation, 21g of xylene was evaporated from the reaction solution, the concentrated solution was stirred and crystallized in an ice-water bath, and 11.8g of o-hydroxybenzonitrile was obtained by filtration, with a content of 98.3% (liquid chromatography external standard method, the same below), and the yield 97.5%.
Embodiment 2
[0017] 13.7g (0.1mol) o-hydroxybenzamide, 68.5g (0.65mol) xylene, 0.041g (4.7×10 -4 mol) dioxane was added to a 100mL three-necked flask with a stirring and reflux tube, heated to reflux, and 11.9g (0.12mol) phosgene was introduced within 3hr.
[0018] After 0.5 hr of heat preservation, 34 g of xylene was evaporated from the reaction solution, the concentrated solution was stirred and crystallized in an ice-water bath, and 11.6 g of o-hydroxybenzonitrile was obtained by filtration, with a content of 98.9% and a yield of 96.4%.
Embodiment 3
[0020] 13.7g (0.1mol) o-hydroxybenzamide, 108g (1.02mol) xylene, 0.068g (7.7×10 -4 mol) dioxane was added to a 100mL three-necked flask with a stirring and reflux tube, heated to reflux, and 11.9g (0.12mol) phosgene was introduced within 4hr.
[0021] After 0.5 hr of heat preservation, 55 g of xylene was evaporated from the reaction solution, the concentrated solution was stirred and crystallized in an ice-water bath, and 11.5 g of o-hydroxybenzonitrile was obtained by filtration, with a content of 99.2% and a yield of 95.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com