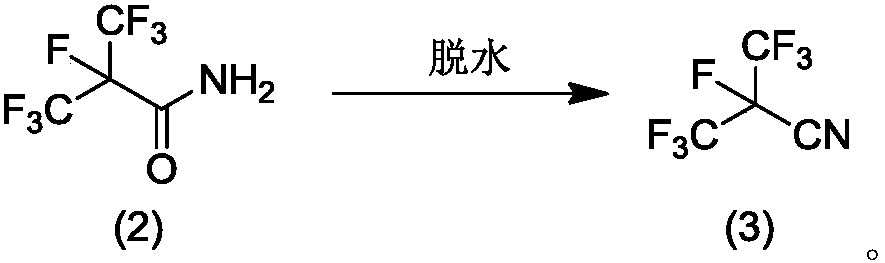
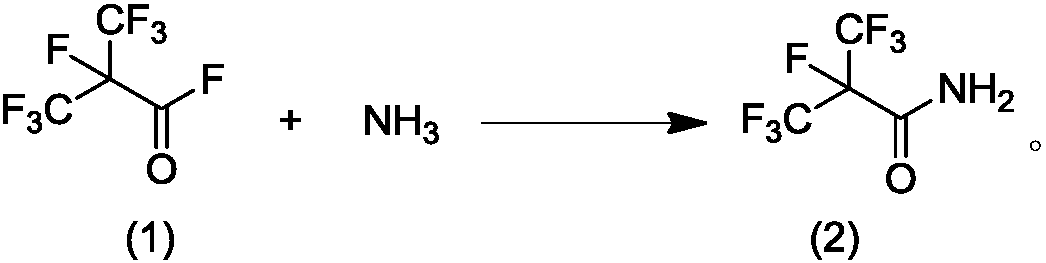Method for preparing perfluoro-isobutyronitrile and intermediates thereof
A technology of perfluoroisobutyronitrile and perfluoroisobutyramide is applied in the field of preparation of perfluoroisobutyronitrile, which can solve the problems of difficult treatment and industrial application of trifluoroacetic acid pyridinium salt, and achieves reduction of three wastes and reaction operation. Simple, novel raw material route effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
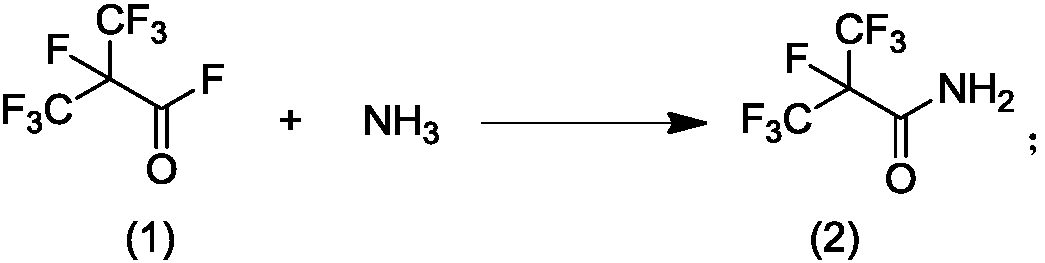
[0047] Example 1: Preparation of perfluoroisobutyramide
[0048] At 0°C, 80 grams of perfluoroisobutyryl fluoride and 12 grams of ammonia gas were introduced into 200 grams of acetonitrile under stirring from two pipelines. The reaction vessel was a dry 500 ml four-necked flask. During the reaction, keep the internal temperature of the solution not exceeding 25°C. After the gas was passed through, stirring was continued for 2 hours. Dissolve the resulting reaction liquid in water and use Na 2 CO 3 After the treatment is made basic, acetonitrile is removed under reduced pressure. The remaining aqueous phase was extracted with dichloromethane or ethyl acetate, separated, dried, filtered, and evaporated to obtain 67.2 g of perfluoroisobutyramide. The yield was 85%.
Embodiment 2
[0049] Example 2: Preparation of perfluoroisobutyramide
[0050] At -10°C, 50 g of perfluoroisobutyryl fluoride and 7.5 g of ammonia gas were passed into 100 g of acetonitrile under stirring, and the reaction vessel was a dry 250 ml four-necked flask. The rest of the operation is the same as in Example 1, and 36.0 g of perfluoroisobutyramide is obtained. The yield was 73%.
Embodiment 3
[0051] Example 3: Preparation of perfluoroisobutyramide
[0052] At 0°C, 50 g of perfluoroisobutyryl fluoride and 12 g of ammonia gas were passed into 200 g of acetonitrile under stirring, and the reaction vessel was a dry 500 ml four-necked flask. The other operations were the same as in Example 1, and 40.5 g of perfluoroisobutyramide was obtained. The yield was 82%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com