Method and device for producing hexamethylenediamine from caprolactam
A technology of caprolactam and hexamethylenediamine, applied in separation methods, bulk chemical production, chemical instruments and methods, etc., can solve the problems of no industrial application value, many by-products, high reaction temperature, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Production of hexamethylenediamine from caprolactam
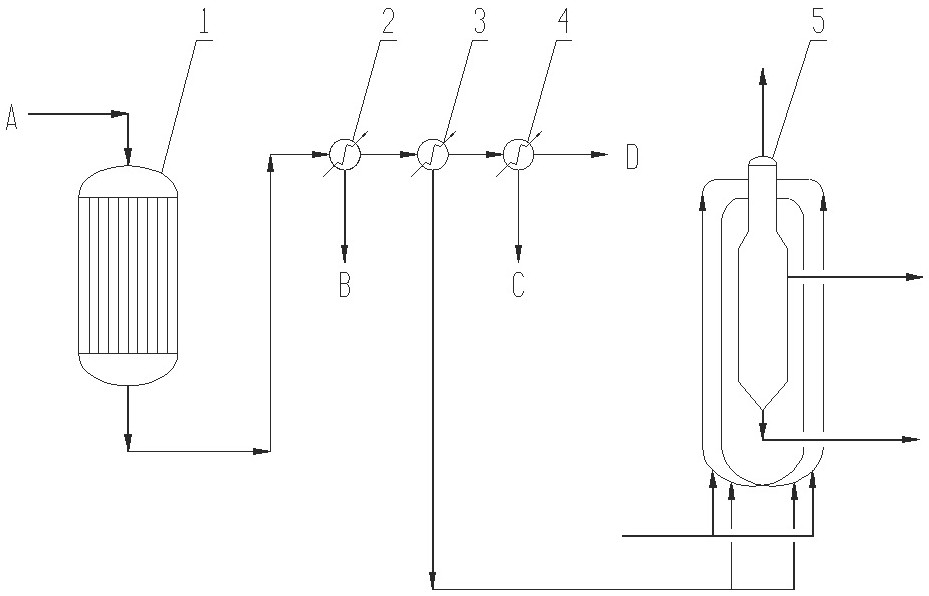
[0056] Evaporate caprolactam in the evaporator, mix it with hot ammonia gas at a molar ratio of 1:30, and enter the fixed-bed reactor filled with magnesium phosphate catalyst. The temperature is controlled at 300-400°C, and the reaction pressure is 0-1.0MPa; 720~3600h -1 ;
[0057] The ammoniated dehydration reaction product obtained above is subjected to primary condensation, cooled to 300-315°C, and the heavy component is separated; then the primary condensed gas phase is subjected to secondary condensation, cooled to 140-210°C, and the caprolactam, 6- The condensate of aminocapronitrile; the gas phase of the secondary condensation is condensed in the third stage, cooled to 40-60°C, and the water is separated, and the ammonia gas in the gas phase is returned to the ammoniation dehydration reaction as a raw material for reuse;
[0058] The caprolactam and 6-aminocapronitrile condensate obtained by the secondary co...
Embodiment 2
[0063] Production of hexamethylenediamine from caprolactam
[0064] Evaporate caprolactam in the evaporator, mix it with hot ammonia gas at a molar ratio of 1:30, and enter the fixed-bed reactor filled with magnesium phosphate catalyst. The temperature is controlled at 300-400°C, and the reaction pressure is 0-1.0MPa; the gas phase space velocity is 730~3600h -1 ;
[0065] The ammoniated dehydration reaction product obtained above is subjected to primary condensation, cooled to 300-315°C, and the heavy component is separated; then, the primary condensed gas phase is subjected to secondary condensation, cooled to 140-210°C, and the caprolactam, 6- The condensate of aminocapronitrile; the gas phase of the secondary condensation is condensed in the third stage, cooled to 40-60°C, and the water is separated, and the ammonia gas in the gas phase is returned to the ammoniation dehydration reaction for reuse as a raw material;
[0066] The caprolactam and 6-aminocapronitrile conden...
Embodiment 3
[0071] Production of hexamethylenediamine from caprolactam
[0072] Evaporate caprolactam in the evaporator, mix it with hot ammonia gas at a molar ratio of 1:30, and enter the fixed-bed reactor filled with magnesium phosphate catalyst. The temperature is controlled at 300-400°C, and the reaction pressure is 0-1.0MPa; 730~3600h -1 ;
[0073] The ammoniated dehydration reaction product obtained above is subjected to primary condensation, cooled to 300-315°C, and the heavy component is separated; then, the primary condensed gas phase is subjected to secondary condensation, cooled to 140-180°C, and the caprolactam, 6- The condensate of aminocapronitrile; the gas phase of the secondary condensation is condensed in the third stage, cooled to 40-60°C, and the water is separated, and the ammonia gas in the gas phase is returned to the ammoniation dehydration reaction for reuse as a raw material;
[0074] The caprolactam and 6-aminocapronitrile condensate obtained by the secondary c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com