Process for producing p-phenyl cyanophenyl
A technology of p-benzonitrile and biphenylcarboxamide, applied in the field of preparation of p-benzonitrile, can solve problems such as high cost and pollution, and achieve the effects of low cost, overcoming high price and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
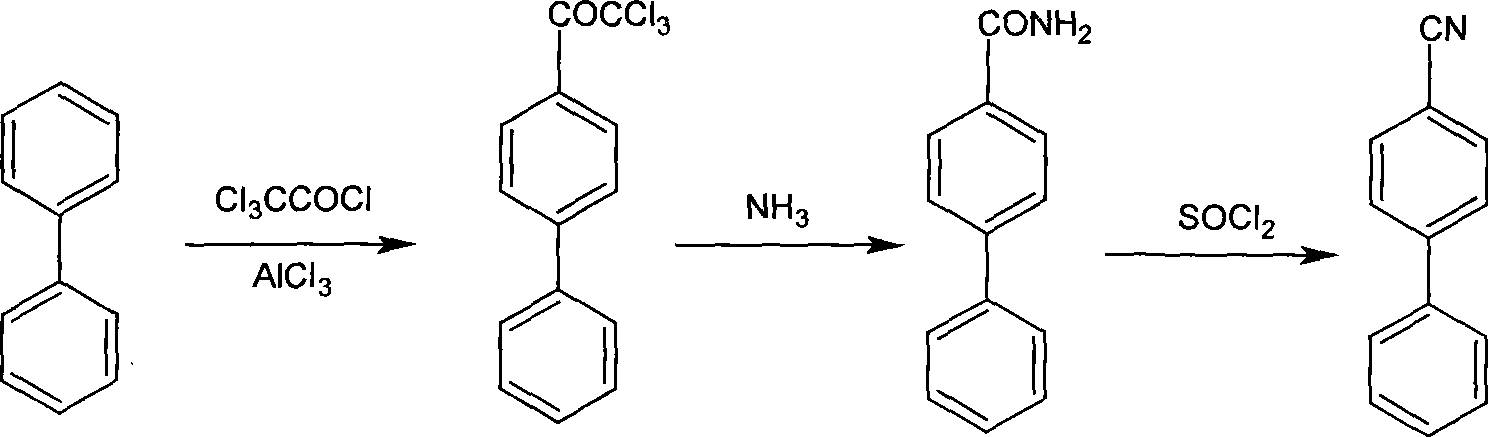
[0048] Add 50kg of biphenyl, 80kg of trichloroacetyl chloride and 400kg of o-dichlorobenzene into the dry reactor, stir to dissolve, add 45kg of anhydrous zinc chloride, a large amount of gas escapes, and control the reaction temperature at 25-30°C under an ice-water bath. After the reaction of the raw materials was complete, the reaction solution was poured into ice water under stirring, extracted and washed, and then concentrated to obtain 92.5 kg of 4-trichloroacetylbiphenyl (95% yield).
[0049] Add 92.5kg of 4-trichloroacetylbiphenyl and 250kg of o-dichlorobenzene into another reactor, stir to dissolve, pass ammonia gas and control the reaction temperature at 20-30°C, a white solid is precipitated, and after the raw materials are completely reacted , filtered, washed, and dried to obtain 59.1 kg of 4-biphenylcarboxamide (97% yield).
[0050]Add 59.1kg of 4-biphenylcarboxamide and 450kg of acetonitrile into the dry reactor, add 88.5kg of thionyl chloride and 1.5kg of catal...
Embodiment 2
[0053] Add 50kg of biphenyl, 80kg of trichloroacetyl chloride and 500kg of dichloromethane into the dry reactor, stir to dissolve, add 50kg of anhydrous aluminum trichloride, a large amount of gas escapes, control the reaction temperature in an ice-water bath at 10-15°C, After the raw materials have reacted completely, the reaction solution is poured into ice water under stirring, extracted and washed, and then concentrated to obtain 93.4 kg of 4-trichloroacetylbiphenyl (96% yield).
[0054] Add 93.4kg of 4-trichloroacetylbiphenyl and 300kg of tetrahydrofuran into another reactor, stir to dissolve, pass ammonia gas and control the reaction temperature at 5-15°C, a white solid is precipitated, after the raw materials are completely reacted, filter and wash After drying, 60.2 kg of 4-biphenylcarboxamide was obtained (yield 98%).
[0055] Add 60.2kg of 4-biphenylcarboxamide and 520kg of dichloroethane into the dry reactor, and add 100.7kg of phosphine oxychloride and 2.5kg of cat...
Embodiment 3
[0058] Add 50kg of biphenyl, 85kg of trichloroacetyl chloride and 450kg of dichloroethane into the dry reaction kettle, stir to dissolve, add 55kg of anhydrous aluminum trichloride, a large amount of gas escapes, control the reaction temperature in an ice-water bath at 20-25oC, After the reaction of the raw materials is complete, the reaction solution is poured into ice water under stirring, extracted, washed with water, and separated to obtain a dichloroethane solution of 4-trichloroacetylbiphenyl, which is directly used in the next reaction without Further purification.
[0059] Add the above-mentioned 4-trichloroacetylbiphenyldichloroethane solution into another reaction kettle, under stirring, pass ammonia gas and control the reaction temperature at 15-25oC, a white solid is precipitated, and after the raw materials are completely reacted, the temperature of the system is raised to 90oC, remove the moisture in the reaction solution by azeotropic removal, and remove the dis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com