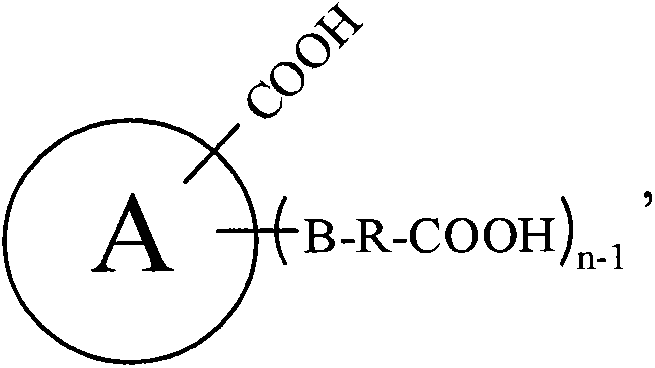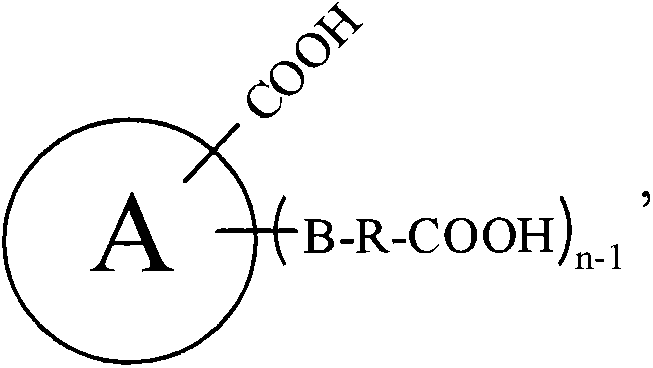Preparation methods of nitrile and corresponding amine
A manufacturing method and amide technology, applied in chemical instruments and methods, dehydration preparation of carboxylic acid amides, preparation of organic compounds, etc., can solve problems such as long reaction time, environmental pressure, reaction material loss and side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0123] The following examples will be used to illustrate the present invention in further detail, but the present invention is not limited to these examples.
[0124] Preparation example of amide intermediate
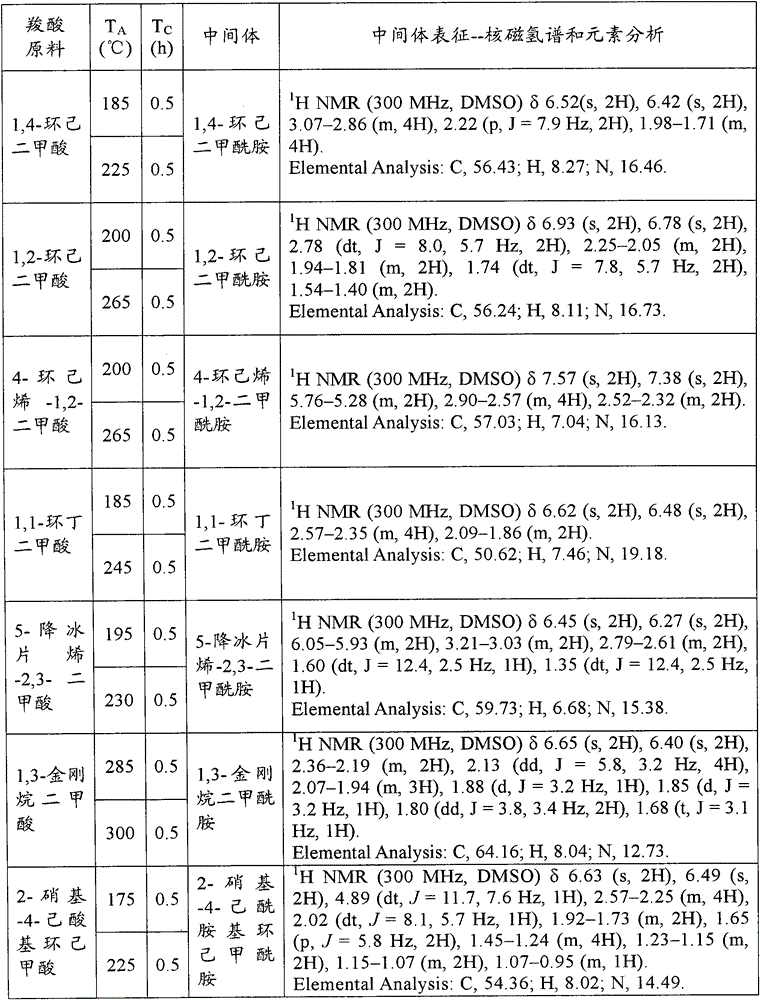
[0125] Add 500g carboxylic acid raw material (chemically pure) in 1L open reactor, open and stir (600r / min), constantly pass into ammonia gas (chemically pure, water content is 5.1wt% in the carboxylic acid raw material from the reactor bottom, flow rate is 100g / min). Make the reaction at the reaction temperature T A T C After 1 hour, the ammonia gas was stopped. The contents of the reactor were sampled for 1H NMR and elemental analysis to characterize the amide intermediate. The specific reaction conditions and characterization results are shown in the following Table A-1, Table A-2, Table A-3, Table A-4 and Table A-5. These characterization results show that the obtained amide intermediate has extremely high purity (over 99%).
[0126] Table A-1
[0127]
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com