Method for preparing 6-aminocapronitrile by gas phase method
A technology of aminocapronitrile and gas phase method, applied in the field of preparing 6-aminocapronitrile by gas phase method, can solve the problems of unsatisfactory reaction effect, low reaction conversion rate and the like, and is suitable for large-scale popularization and application and has high reaction conversion rate. , the effect of simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
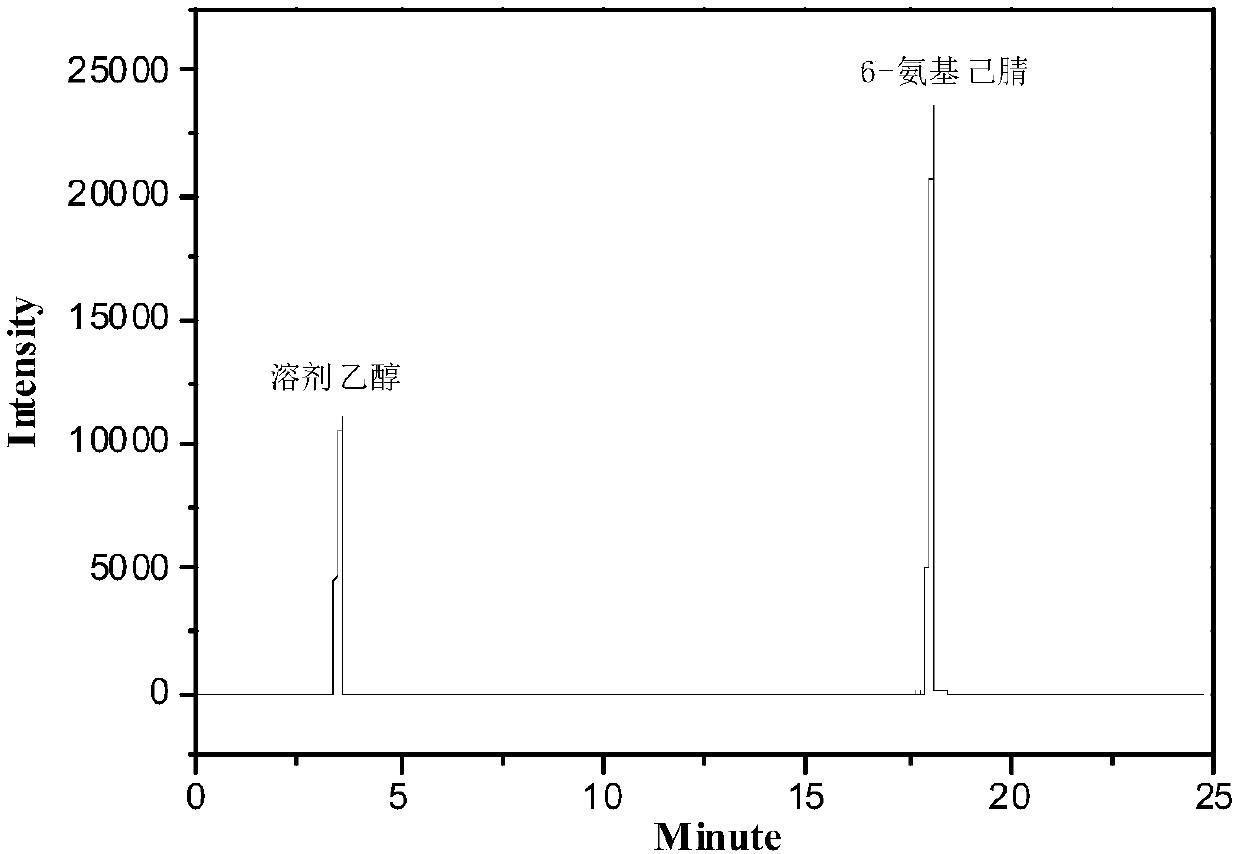
[0029] Fully mix 6 g / h of caprolactam vapor with 18 L / h of hot ammonia gas (the mass ratio of ammonia gas to CPL is 2.3), and the temperature of ammonia gas is 270°C. The mixed steam of caprolactam and ammonia is contacted with 1g catalyst, the contact time is 0.1s, the reaction temperature is 300°C, the reaction pressure is 0.1mpa, and the catalyst is silicon dioxide. The reaction conversion rate is 57.3%, and the selectivity is 97.8%. The effluent of the ammoniation reaction is deaminated, dehydrated, and 99.6% of 6-aminocapronitrile can be obtained after rectification under reduced pressure.
[0030] In this embodiment, the bottom temperature of the vacuum distillation tower is 150° C., and the vacuum degree is 2 torr. When the tower top temperature is 20° C., ammonia and water are evaporated; when the tower top temperature is 130° C., 6-aminocapronitrile is evaporated.
Embodiment 2
[0032] Fully mix 6 g / h of caprolactam vapor with 48 L / h of hot ammonia gas (the mass ratio of ammonia gas to CPL is 6), and the temperature of ammonia gas is 320°C. The mixed steam of caprolactam and ammonia is contacted with 5g catalyst, the contact time is 0.5s, the reaction temperature is 340°C, the reaction pressure is 0.5mpa, and the catalyst is activated alumina. The reaction conversion rate is 78%, and the selectivity is 98.3%. The effluent of the ammoniation reaction is deaminated and dehydrated, and 99.8% of 6-aminocapronitrile can be obtained after rectification under reduced pressure.
[0033] In this embodiment, the temperature at the bottom of the vacuum distillation tower is 152°C, and the vacuum degree is 2.4torr. When the temperature at the top of the tower is 25°C, ammonia and water are evaporated; when the temperature at the top of the tower is 131°C, 6-aminocapronitrile is evaporated. .
Embodiment 3
[0035] Fully mix 6 g / h of caprolactam vapor with 90 L / h of hot ammonia gas (the mass ratio of ammonia gas to CPL is 11.4), and the temperature of ammonia gas is 350°C. The mixed steam of caprolactam and ammonia is contacted with 10g of catalyst, the contact time is 1s, the reaction temperature is 350°C, the reaction pressure is 0.3mpa, and the catalyst is cerium oxide. The conversion rate of the reaction is 85%, and the selectivity is 98.5%. The effluent of the ammoniation reaction is deaminated and dehydrated, and 99.8% of 6-aminocapronitrile can be obtained after rectification under reduced pressure.
[0036] In this embodiment, the temperature at the bottom of the vacuum distillation tower is 155°C, and the vacuum degree is 2.8 torr. When the temperature at the top of the tower is 28°C, ammonia and water are evaporated; when the temperature at the top of the tower is 135°C, 6-aminocapronitrile is evaporated. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com