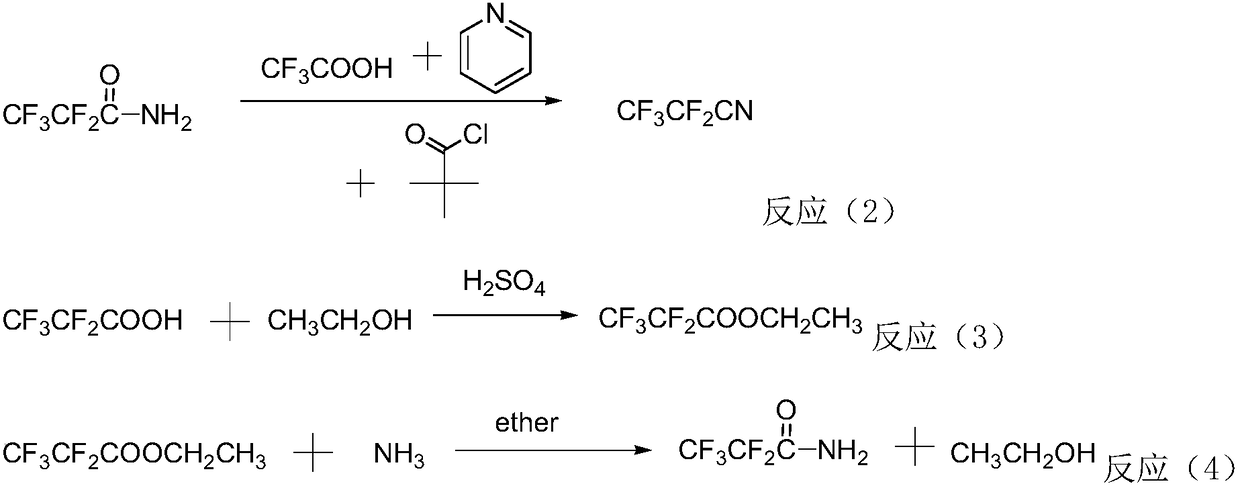
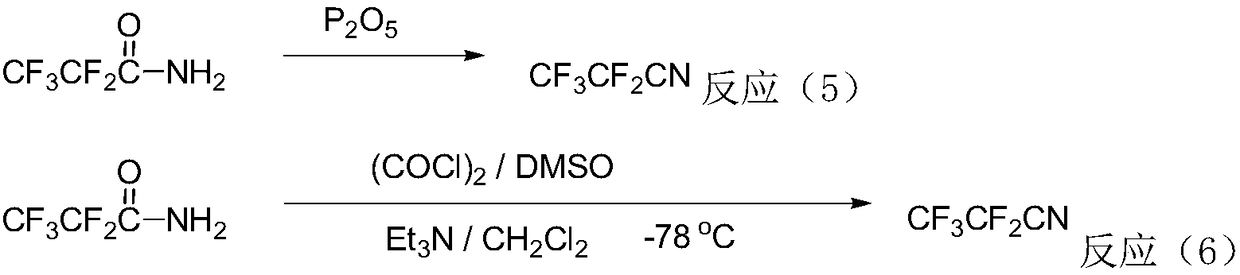
Method for preparing perfluorinated nitrile through gas phase catalysis
A technology for catalytic preparation and perfluoronitrile, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylic acid amides, etc., can solve the problems of unobtainable raw materials, low conversion efficiency, difficult continuous production, etc., and achieve a synthetic route. Novel, high overall yield, and the effect of reducing the cost of industrial synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] The reaction was carried out in a tube reactor made of Inconium alloy with an outer diameter of 1 / 2 inch and an inner volume of 30 milliliters. The reaction conditions are: the reaction temperature is 150°C, the molar ratio of 2,2,3-trifluoro-3-(trifluoromethyl)oxirane to methylamine is 1:3, the contact time is 1s, and the reaction pressure is 0.1 MPa. The reactant flows through a bottle of polytetrafluoroethylene material to condense and collect, the solid remains at the bottom of the bottle, and the gas phase is discharged from the system. After 10 hours of reaction, 1946.9 grams of solid pentafluoropropionamide were obtained, with a purity of 99.4% (GC analysis), five The yield of fluoropropionamide was 98.5%.
Embodiment 2
[0058] The reaction was carried out in a tube reactor made of Inconium alloy with an outer diameter of 1 / 2 inch and an inner volume of 30 milliliters. The reaction conditions are: the reaction temperature is 150°C, the molar ratio of 2,2,3-trifluoro-3-(trifluoromethyl)oxirane to methylamine is 1:3, the contact time is 10s, and the reaction pressure is 0.1 MPa. The reactant flows through the bottle of polytetrafluoroethylene material to condense and collect, the solid remains at the bottom of the bottle, and the gas phase is discharged from the system. After 10 hours of reaction, 195.1 grams of solid pentafluoropropionamide were obtained, with a purity of 99.6% (GC analysis), five The yield of fluoropropionamide was 98.9%.
Embodiment 3
[0060] The reaction was carried out in a tube reactor made of Inconium alloy with an outer diameter of 1 / 2 inch and an inner volume of 30 milliliters. The reaction conditions are: the reaction temperature is 150°C, the molar ratio of 2,2,3-trifluoro-3-(trifluoromethyl)oxirane to methylamine is 1:3, the contact time is 100s, and the reaction pressure is 0.1 MPa. The reactant flows through a bottle of polytetrafluoroethylene material to condense and collect, the solid remains at the bottom of the bottle, and the gas phase is discharged from the system. After 10 hours of reaction, 19.5 grams of solid pentafluoropropionamide were obtained, with a purity of 99.8% (GC analysis). The yield of fluoropropionamide was 99.2%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com