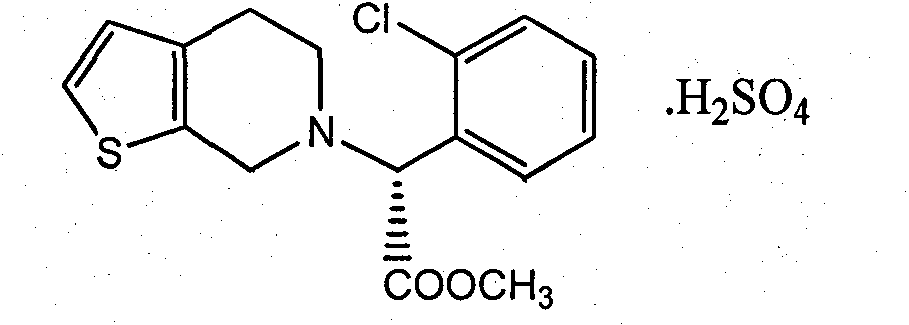
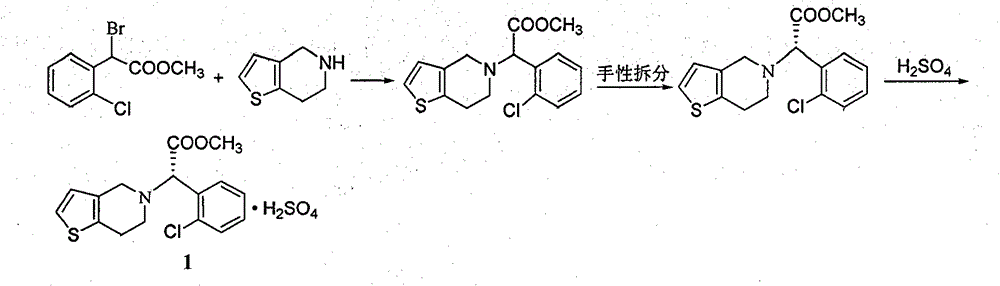
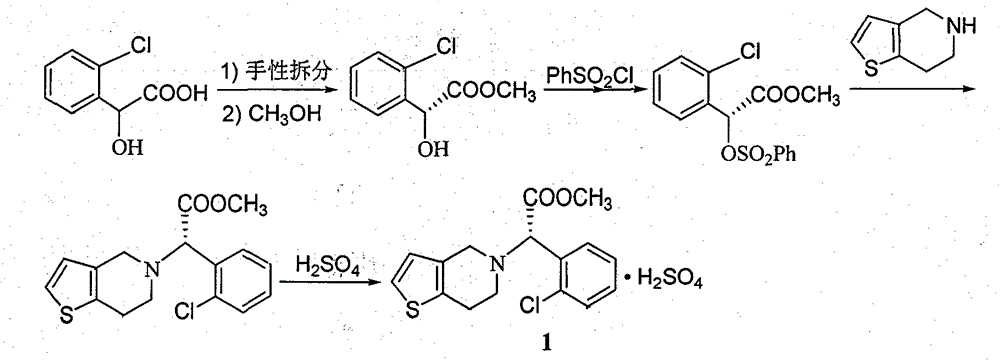
Clopidogrel hydrogen sulfate preparation method
A technology of clopidogrel hydrogen sulfate and concentrated sulfuric acid is applied in the field of preparation of clopidogrel hydrogen sulfate, can solve the problems of long route, limited heating and reflux temperature, etc., and achieves the effects of easy availability of raw materials, reduced cost and short synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The preparation method of clopidogrel hydrogen sulfate of the present invention includes the following steps:
[0036] 1) Weigh 12.6 g of 2-thiophene acetaldehyde and 20.0 g of S-o-chlorophenylglycine methyl ester, add them to a 250 mL three-necked reaction flask, add 100 mL of organic solvent toluene and 0.4 g of catalyst ammonium acetate, heat and stir to reflux for reaction. The temperature is 110°C. TLC tracking monitoring showed that the raw materials disappeared. The reaction solution was cooled to 25° C., 100 mL of water was added, and the mixture was allowed to stand for separation after stirring. The toluene layer was dried with anhydrous sodium sulfate. Then it was filtered, and the filtrate was evaporated to dryness under reduced pressure to obtain a crude solid imine intermediate product, which was recrystallized with ethanol to obtain 28.7 g of pure imine intermediate, with a yield of 93.2%.
[0037] 2) Take 15.4g of the imine intermediate obtained in step 1) i...
Embodiment 2
[0041] The preparation method of clopidogrel hydrogen sulfate of the present invention includes the following steps:
[0042] 1) Weigh 12.6g of 2-thiopheneacetaldehyde and 20.0g of S-o-chlorophenylglycine methyl ester, add them to a 250mL three-necked reaction flask, add 100mL of solvent chloroform and 0.6g of catalyst ammonium chloride, heat and stir to reflux, reaction temperature The temperature was 60°C and TLC tracking monitoring showed that the raw materials disappeared. The reaction solution was cooled to 20°C, 100 mL of water was added, and the mixture was stirred and left to stand for separation. The chloroform layer was dried with anhydrous sodium sulfate. Then filtered, the filtrate was evaporated to dryness under reduced pressure to obtain a crude solid imine intermediate product, which was recrystallized with ethanol to obtain 27.9 g of a pure imine intermediate, with a yield of 90.6%.
[0043] 2) Take 15.4g of the imine intermediate obtained in step 1) and add it to a...
Embodiment 3
[0047] The preparation method of clopidogrel hydrogen sulfate of the present invention includes the following steps:
[0048] 1) Weigh 12.6g of 2-thiopheneacetaldehyde and 20.0g of S-o-chlorophenylglycine methyl ester, add them to a 250mL three-necked reaction flask, add 100mL of solvent dichloromethane and 0.4g of catalyst ammonium acetate, heat and stir to reflux, react The temperature was 40°C and TLC tracking monitoring showed that the raw materials disappeared. The reaction solution was cooled to 30°C, 100 mL of water was added, and the mixture was stirred and left to stand for separation. The methylene chloride layer was dried with anhydrous sodium sulfate. Then it is filtered, and the filtrate is evaporated to dryness under reduced pressure to obtain a crude solid imine intermediate product. The crude product is recrystallized from ethanol to obtain 28.8 g of pure imine intermediate with a yield of 93.5%.
[0049] 2) Weigh 15.4g of the imine intermediate obtained in step 1) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com