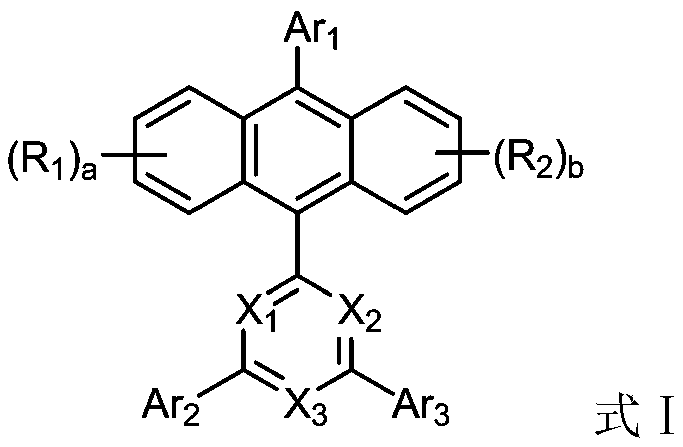
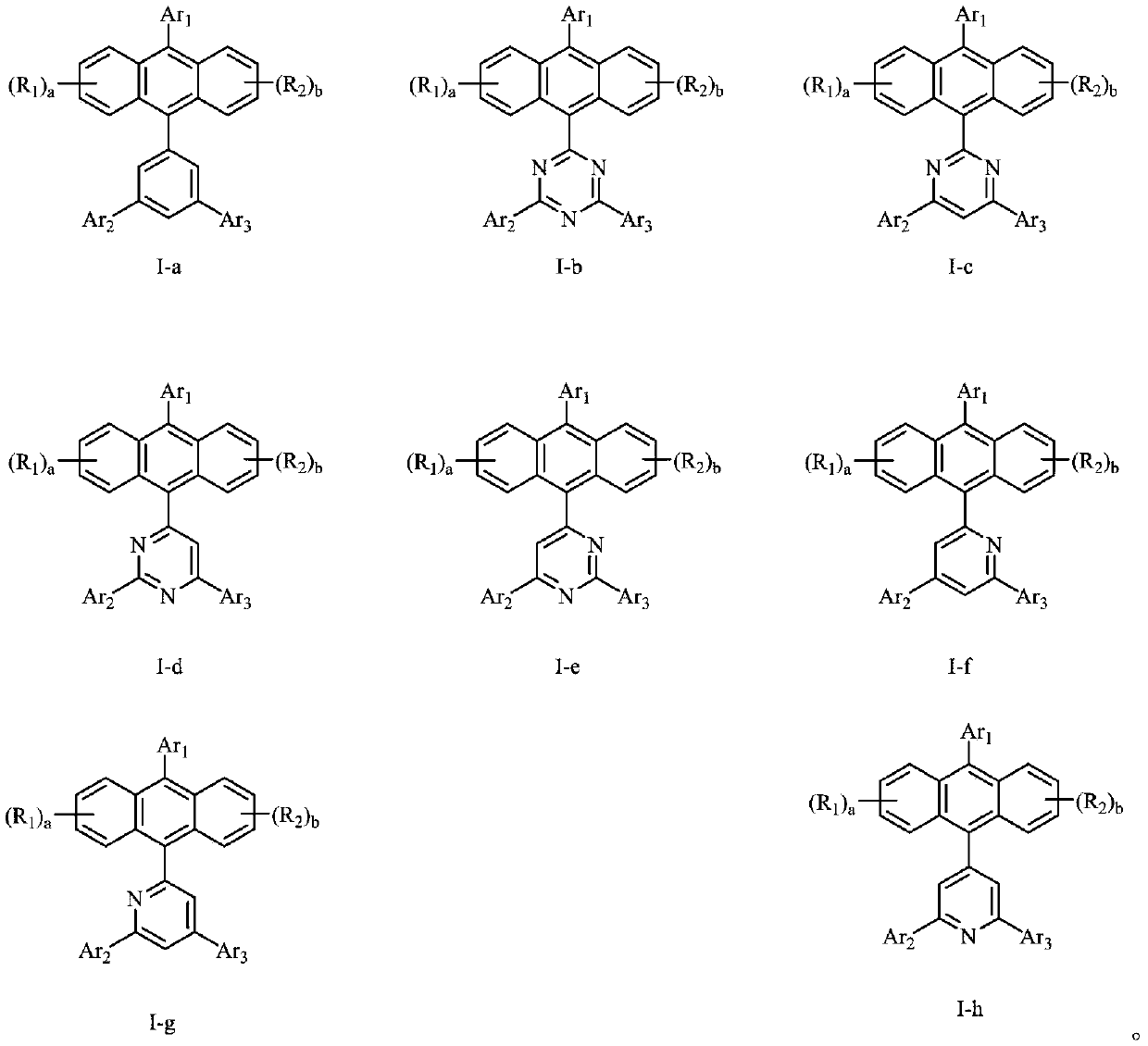
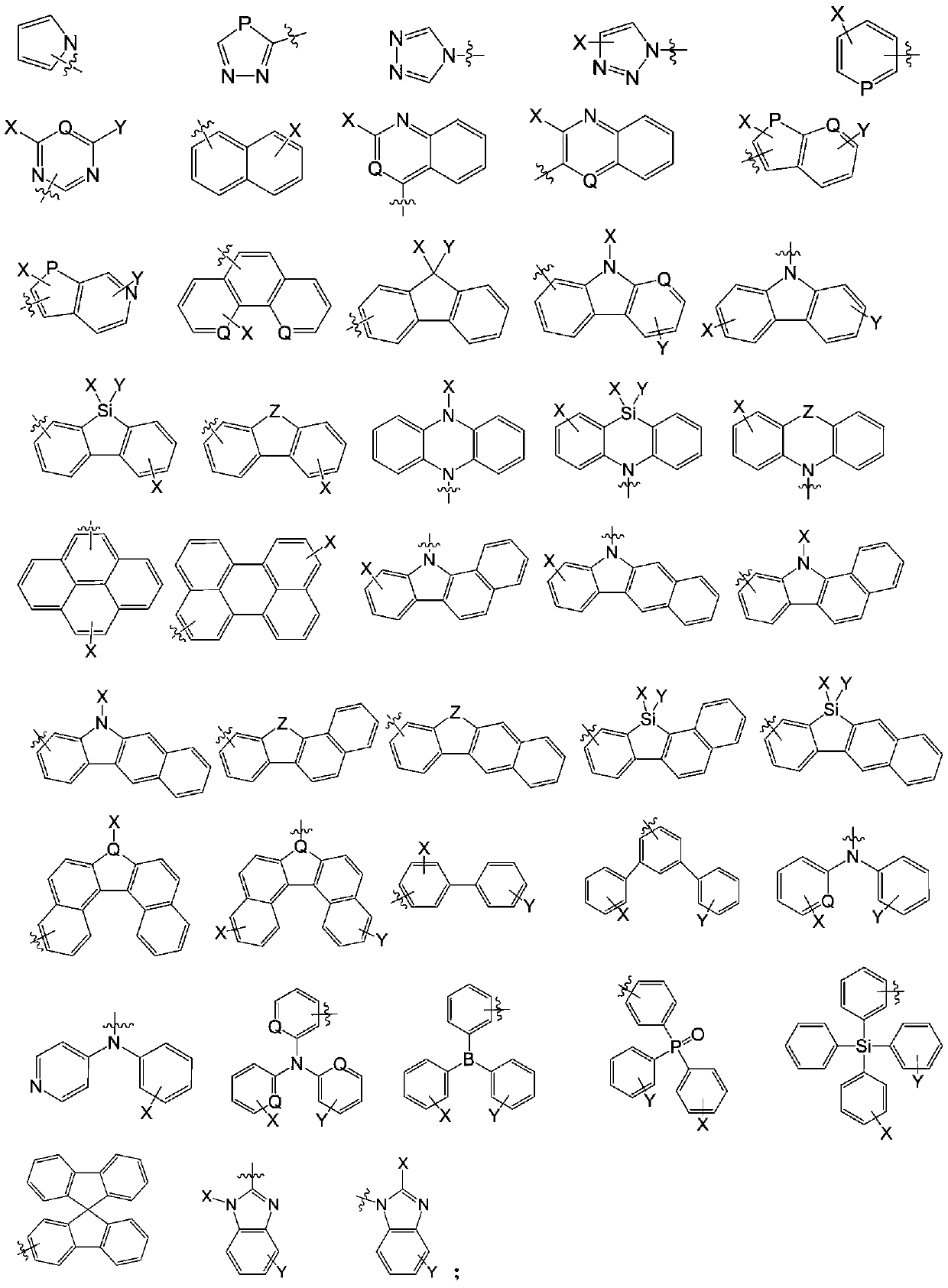
Anthracene organic luminescence compound, and preparation method and application thereof
A light-emitting compound, compound technology, applied in the direction of silicon organic compounds, organic chemistry, chemical instruments and methods, etc., can solve problems such as restricting applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0110] Synthesis of Intermediate C-1:
[0111]
[0112] Reaction process: Add tris(dibenzylideneacetone)dipalladium (20.0g, 0.67mmol) and tricyclohexylphosphine (0.62g, 2.20mmol) into 500ml of 1,4-dioxane solvent, activate at room temperature 30 minutes. The air was replaced three times with nitrogen, Intermediate A-2 (20.0 g, 73.23 mmol), pinacol diboronate (20.46 g, 80.55 mmol) and potassium acetate (14.37 g, 146.46 mmol) were added. The air was replaced with nitrogen three times again, heated to 100° C. and stirred overnight under the protection of nitrogen. The end point of the reaction was monitored by TLC.
[0113] Processing: TLC monitoring. After the reaction was completed, it was cooled to room temperature under the protection of nitrogen. The catalyst was removed using celite and the celite was washed with DCM until free of product. The filtrate was distilled under reduced pressure to a small amount, and added dropwise to petroleum ether (300ml) to precipitat...
Embodiment 2
[0119] Synthesis of Intermediate D-2
[0120]
[0121] Reaction process: intermediate B-2 (18.50g, 57.78mmol), intermediate C-2 (34.33g, 57.78mmol), potassium carbonate (15.97g, 115.56mmol) were added to 500ml toluene / ethanol / water (volume ratio Preferably in a mixed solvent of 3:1:1). Replace the air with nitrogen three times, add tetrakis(triphenylphosphine)palladium, replace the air with nitrogen three times again, heat to 90° C. under the protection of nitrogen, and stir overnight for reaction.
[0122] Workup: TLC monitors the reaction. After the reaction was completed, the catalyst was removed using diatomaceous earth, the liquid was separated, the organic phase was retained, and a small amount was distilled under reduced pressure. The resulting crude product was separated by column chromatography (eluent preferably DCM:PE=1:5) to obtain intermediate D-2 (34.78 g, yield 85%).
[0123] The intermediates shown in Table 2 were prepared according to the above method. ...
Embodiment 3
[0129] Synthesis of intermediate G-1
[0130]
[0131] Reaction process: join three (dibenzylideneacetone) dipalladium (0.44g, 0.48mmol) and tricyclohexylphosphine (0.4g, 1.44mmol) in the 1,4-dioxane solvent of 500ml dryness, use Nitrogen replaced the air three times and activated at room temperature for 30 minutes. D-2 (34.0 g, 48.0 mmol), pinacol diboronate (13.41 g, 52.8 mmol) and potassium acetate (9.41 g, 96.0 mmol) were added to the reaction liquid. The air was replaced with nitrogen three times, and the reaction solution was heated to 100°C.
[0132] Workup: TLC monitors the reaction. After the reaction was completed, it was cooled to room temperature under the protection of nitrogen. The catalyst was removed using celite and the filter cake was rinsed with dichloromethane until free of product. Concentrate the filtrate at least a little, and add the concentrate dropwise to petroleum ether while stirring. After the solid was completely precipitated, it was sucti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com