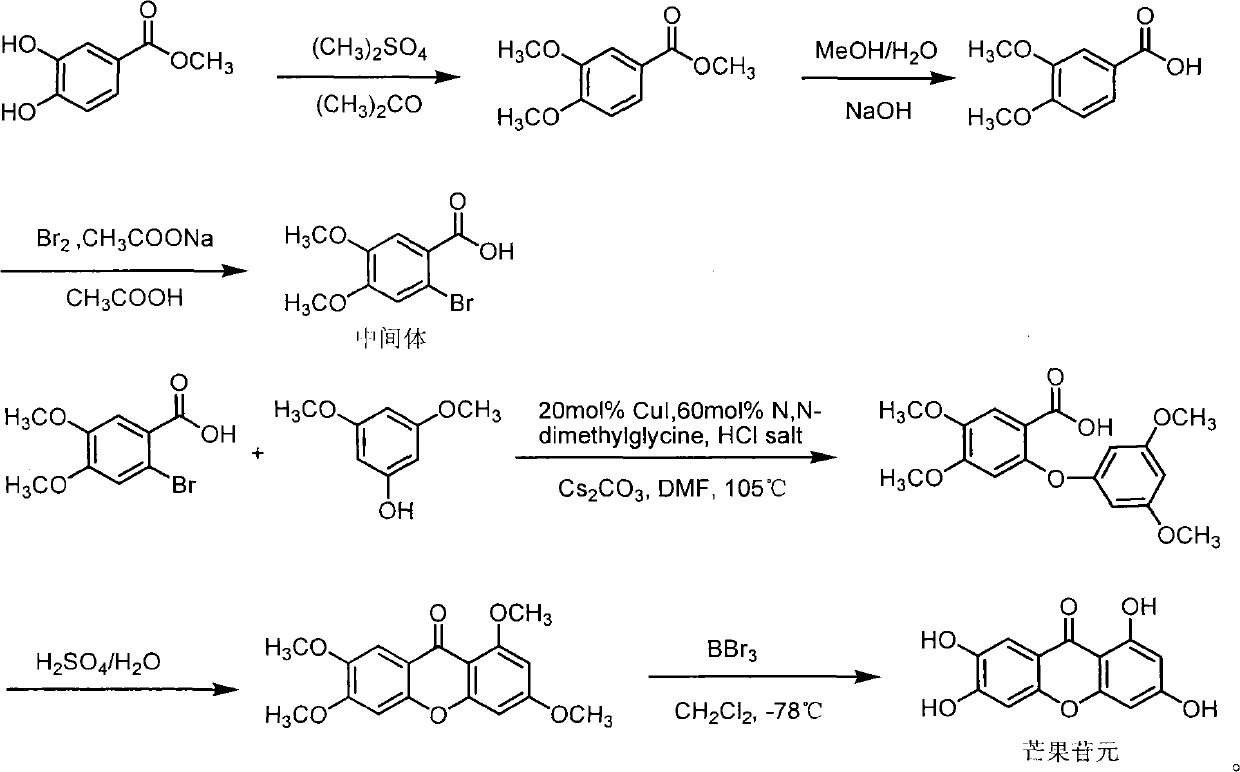
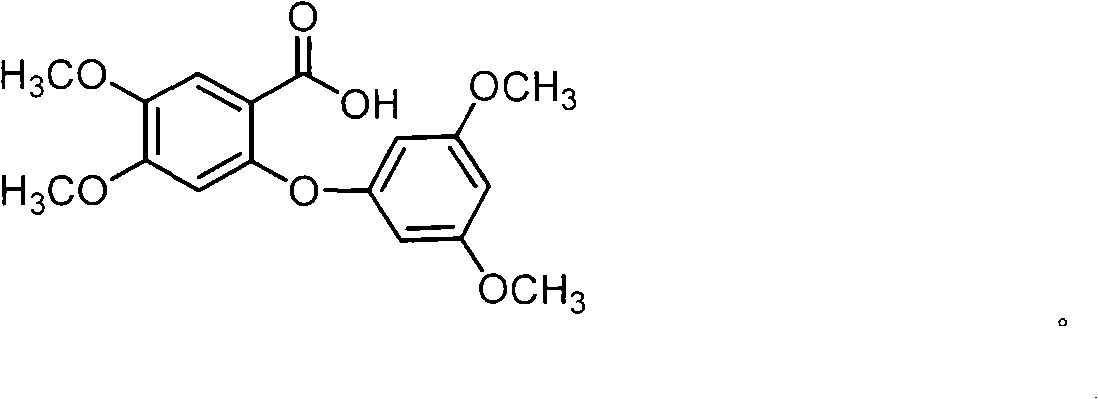
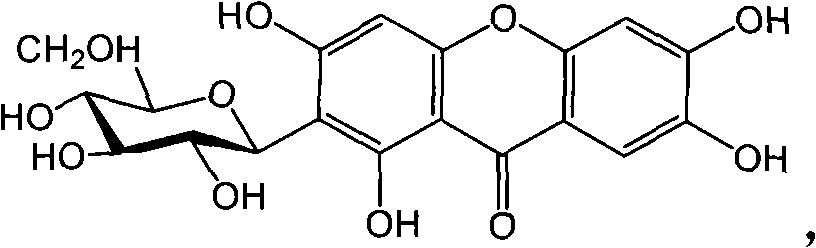
Full chemical synthesis method for mangiferin aglycones
A mangiferin and chemical synthesis technology, applied in the field of total chemical synthesis technology, can solve problems such as complex structure, low yield, and lack of plant resources, and achieve the effects of short synthetic route, mild synthetic reaction conditions, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0020] The present invention is further illustrated below by examples. The examples of the present invention are given only to illustrate the present invention, not to limit the present invention. Therefore, any simple improvement to the present invention under the premise of the method of the present invention belongs to the protection scope of the present invention.
[0021] (1) 3, the synthesis of methyl 4-dimethoxybenzoate
[0022]
[0023] Methyl 3,4-dihydroxybenzoate (65.1g, 0.39mol), anhydrous acetone (P 2 o 5 Drying, 1500ml) and anhydrous potassium carbonate (200g) were placed in a 2L three-necked flask, heated to reflux, and dimethyl sulfate (200ml, 2.07mol) was added dropwise, and the drop was completed within 3 hours. TLC followed the reaction process and reacted after 12 hours Completely, add 10% potassium hydroxide solution (200ml), heat to reflux for 0.5h, cool, filter, wash the filter cake with 800ml acetone, recover acetone under reduced pressure, and ext...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com