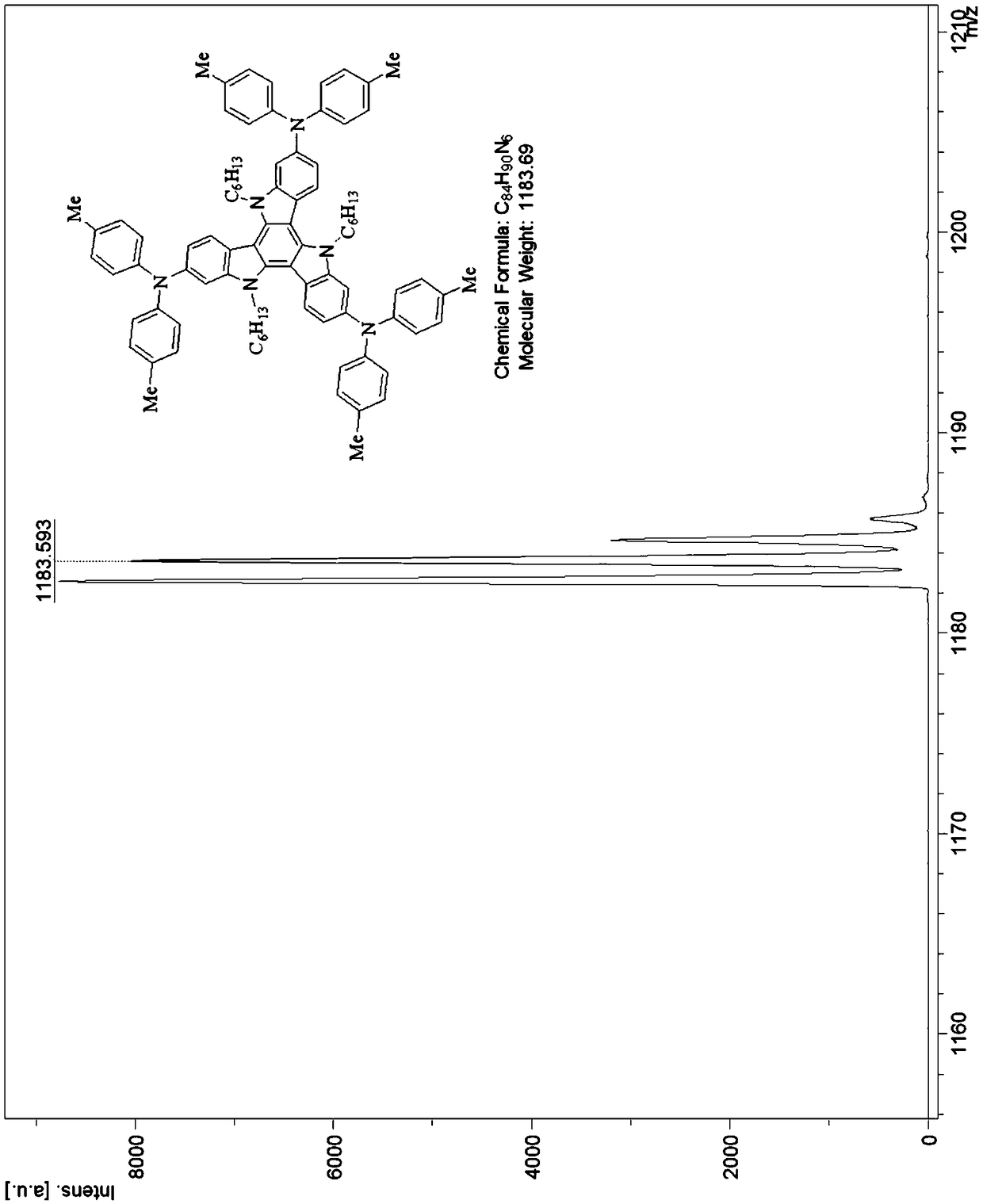
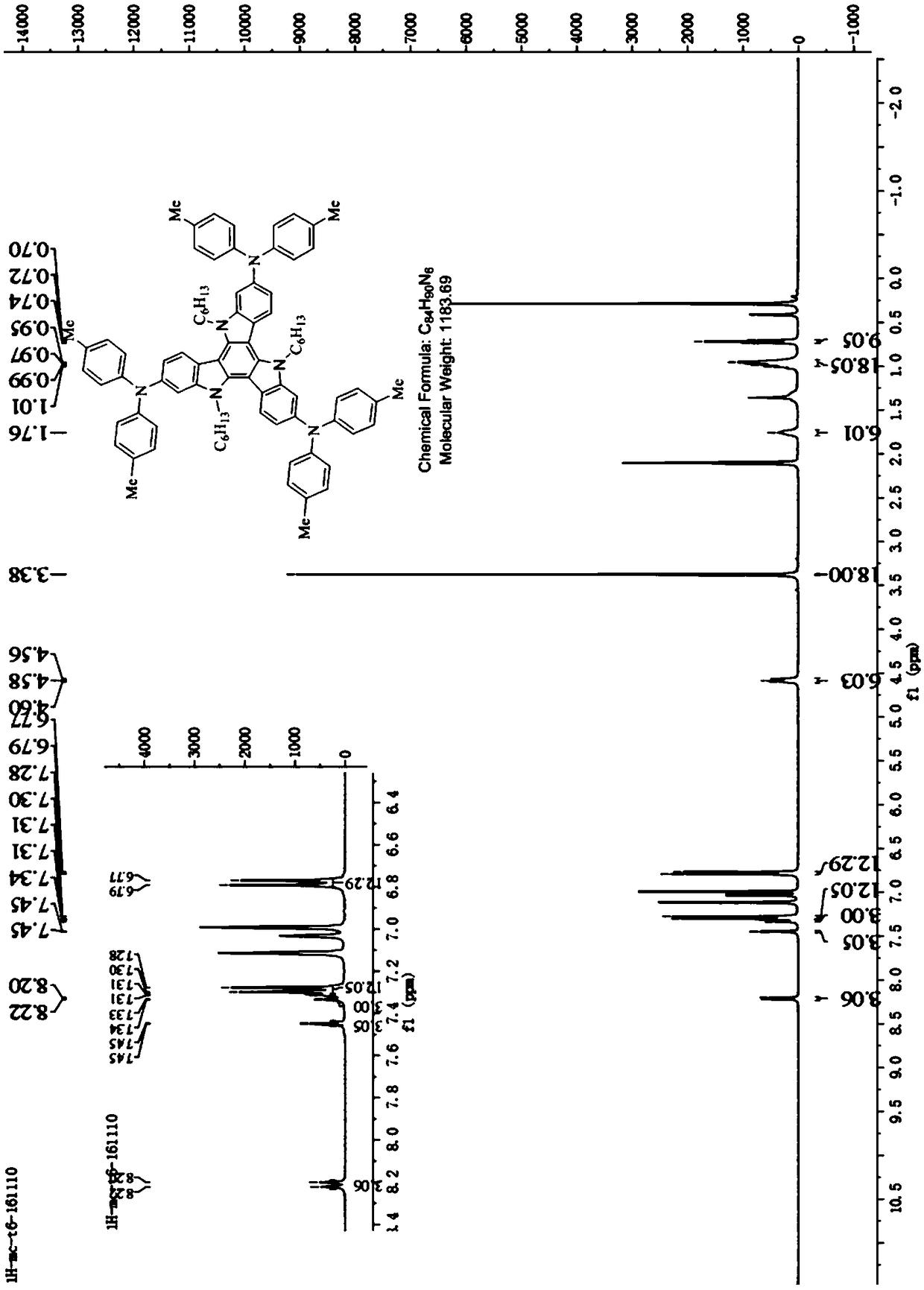
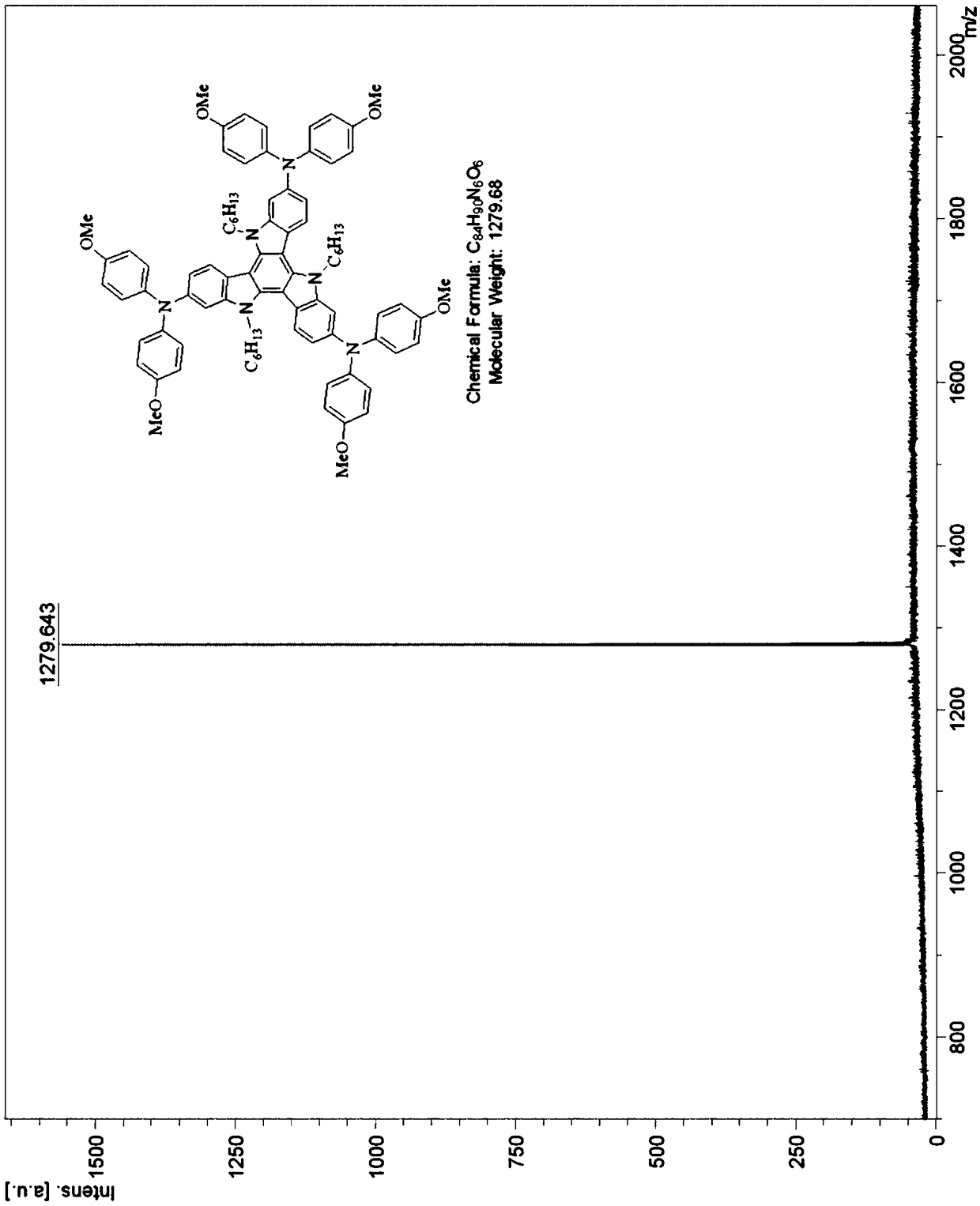
Tricarbazole-aromatic amine derivative cavity transmission material and preparation method and application thereof
A technology of aromatic amine derivatives and hole transport materials, which can be used in semiconductor/solid-state device manufacturing, photovoltaic power generation, electrical components, etc., can solve the problems of poor performance, complex preparation of hole transport materials, high cost, etc., and achieve high yield High, cheap reaction raw materials, easy separation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] A kind of preparation method of tricarbazole-aromatic amine derivative hole transport material of the present invention, comprises the following steps:
[0040] Preparation of compound I: Dissolve compound II, palladium catalyst, any Ar1-Ar6 monoboronic acid or secondary amine and base in toluene under nitrogen protection and light-shielding conditions, and react at a temperature of 60-120°C for 8-72 hours. After the reaction was completed, compound I was purified by column chromatography;
[0041] Wherein, the structure of compound II is shown in formula 3:
[0042]
[0043] Wherein, the mol ratio of compound II and any one of Ar1-Ar6 monoboronic acid or secondary amine is 1:3~1:6; One of them, the molar ratio to Compound II is 0.09:1~0.15:1; the base is potassium carbonate aqueous solution or potassium tert-butoxide, and the molar ratio to Compound II is 4:1~6:1; Compound II The mass volume ratio to toluene is: 15-25 mL of toluene solvent is added for every 500 m...
Embodiment 1
[0048]
[0049] Reaction condition one: under the protection of nitrogen, put 6-bromoindol-2-one (212.0mg, 1.0mmol), potassium carbonate (276mg, 2mmol), tetrabutylammonium bromide (33mg, 0.1mmol) in bis Mouth reaction bottle. Seal the reaction device, change the nitrogen three times, and insert the nitrogen balloon. Inject 22mL of tetrahydrofuran, stir at 85°C for 30min, then slowly inject 2mL of bromohexane, and keep stirring at 80°C for 8h. After the reaction was slowly cooled to room temperature, it was poured into ice water to quench, the organic phase was extracted with dichloromethane, washed three times with deionized water, and the organic phase was dried with anhydrous magnesium sulfate. After suction filtration, the organic phase was concentrated under vacuum to obtain a crude product, which was purified by column chromatography (eluent: dichloromethane (DCM) / petroleum ether (PE) = 1:1) to obtain white waxy solid compound 1 - Hexyl-6-bromo-indolinone (167 mg), y...
Embodiment 2
[0054]
[0055] Reaction condition 1: Put the compound 1-hexyl-6-bromo-indolinone (10g, 33mmol) in a double-necked reaction flask, seal the reaction device, inject 40mL of phosphorus oxychloride, and keep stirring at 100°C for 8h. After the reaction was slowly cooled to room temperature, it was quenched by pouring into ice water, adjusting the Ph to neutral, and suction filtering to obtain a solid crude product, natural. Purification by column chromatography (eluent: DCM / PE=1:10) gave compound II (1.86 mg) as a white solid with a yield of 20.3%.
[0056] Reaction condition 2: Put the compound 1-hexyl-6-bromo-indolinone (10 g, 33 mmol) in a double-necked reaction flask, seal the reaction device, inject 80 mL of phosphorus oxychloride, and keep stirring at 100° C. for 8 h. After the reaction was slowly cooled to room temperature, it was quenched by pouring into ice water, adjusting the Ph to neutral, and suction filtering to obtain a solid crude product, natural. Purified by...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com