Method for synthesizing 6-fluoroindole-3-acetonitrile
A synthesis method and fluoroindole technology are applied in the field of compound synthesis, can solve the problems of product viscosity yield, high environmental pressure, poor operating environment and the like, and achieve the effects of short synthesis route, high reaction yield and convenient control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
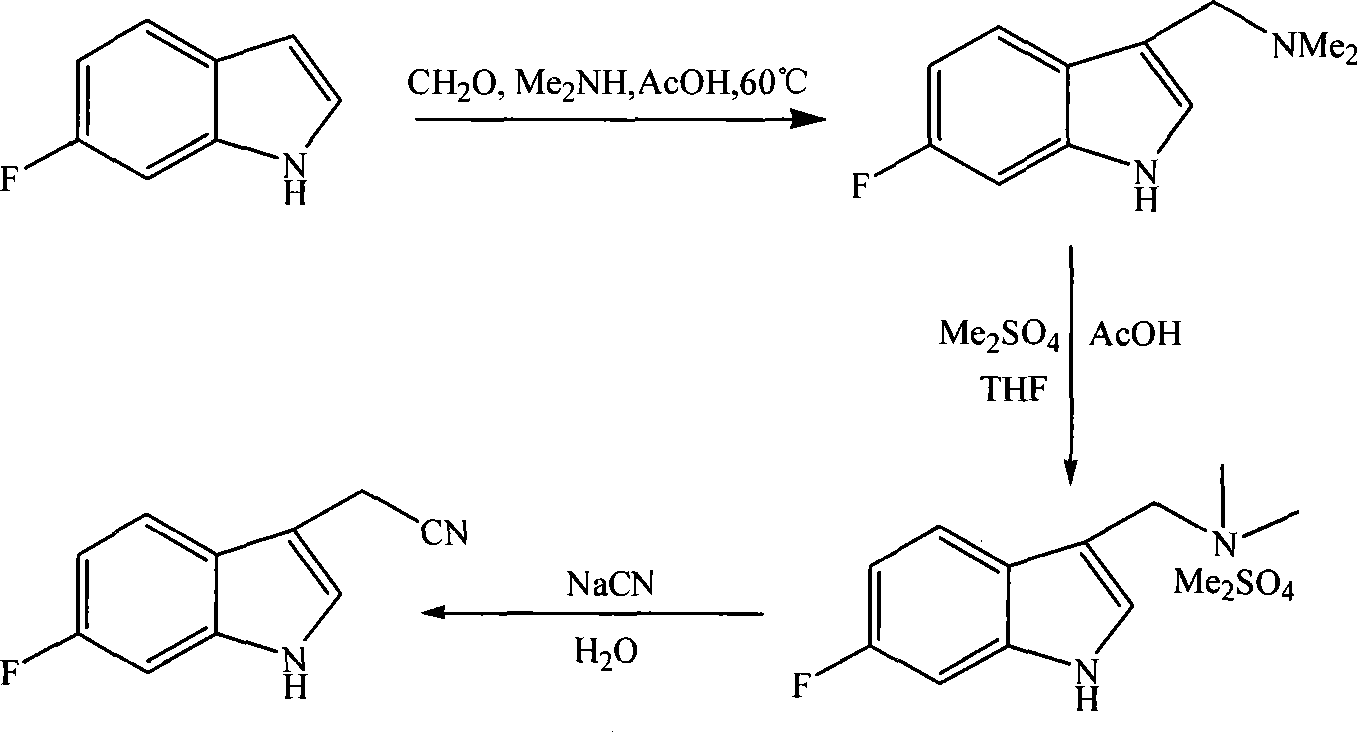
[0022] The synthetic method of 6-fluoroindole-3-acetonitrile is synthesized according to the following operational route:
[0023]
[0024] The specific synthesis process is:
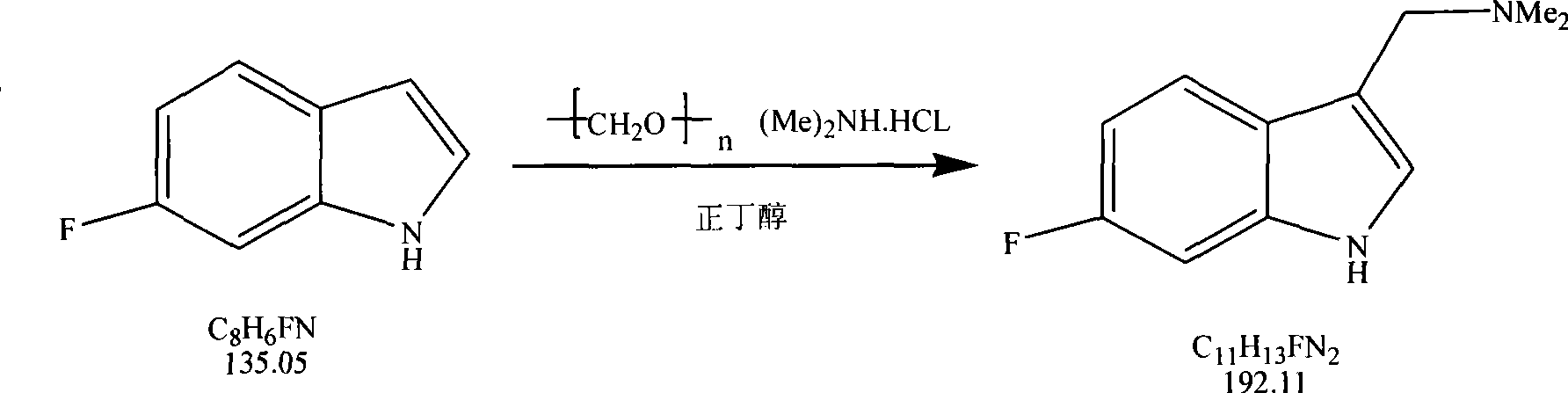
[0025] The first step of synthesizing 6-Fluorophylline:
[0026] Reaction formula
[0027]
[0028] Ratio of raw materials:
[0029] Material name Feeding quality / g The molar ratio of Remark 6-fluoroindole 67.5 1 Industrial Products (98%) Dimethylamine hydrochloride 40.8 1 Industrial Products (99%) Paraformaldehyde 15 1 Industrial Products (99%)
[0031] Experimental steps:
[0032] Add 67.5g (0.5mol) 6-fluoroindole, 40.8g (0.5mol) dimethylamine hydrochloride, 15g (0.5 mol) paraformaldehyde and 170 g of n-butanol, heated to 120° C. to reflux, the solution was purple, and cooled to room temperature after reflux for 2 hours. After the n-butanol was evaporated from the reaction solution, 20% NaOH solut...
Embodiment 2—4
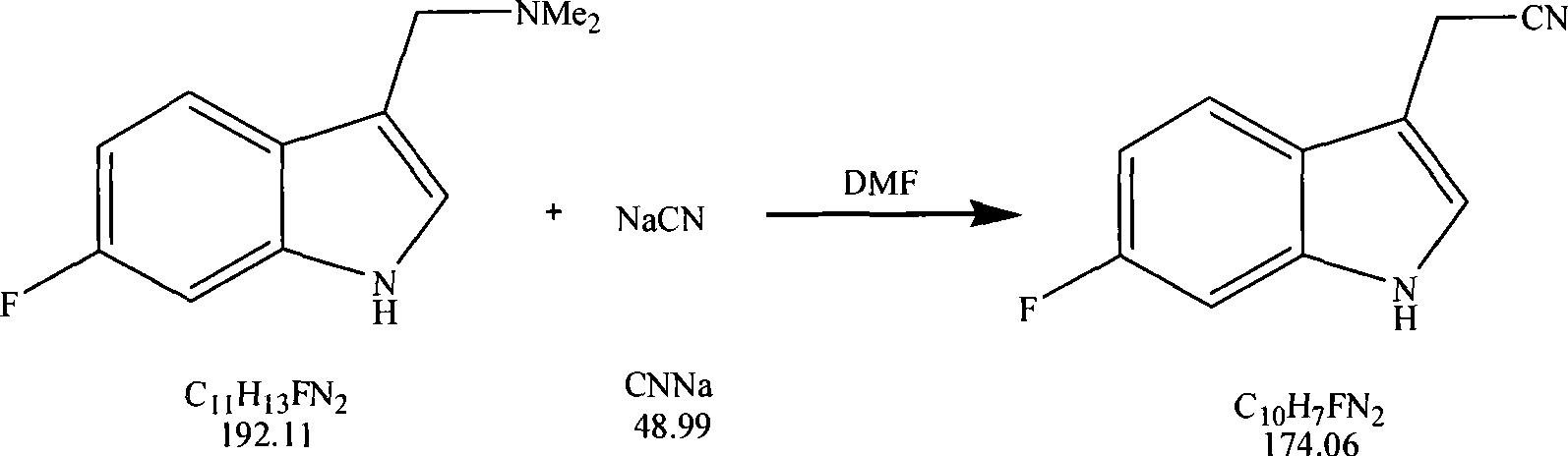
[0041] According to the synthesis method of Example 1, 6-fluoroindole-3-acetonitrile is synthesized, wherein the first step synthesizes 6-fluorodraphylline and the ratio of raw materials:
[0042] Material name Example 2 Example 3 Example 4 6-fluoroindole (mol) 1 1 1 Dimethylamine hydrochloride (mole) 1 1.5 1.3 Paraformaldehyde (mol) 1.1 2 1.5 n-butanol / g 170 180 Isobutanol / g 200
[0043] The second step synthesizes 6-fluoroindole-3-acetonitrile raw material feeding ratio:
[0044] Material name Example 2 Example 3 Example 4 6-Fluorophylline (mol) 1 1 1 Sodium cyanide (mol) 1.2 1.5 2 DMF / g 155 DMSO / g 160 NMP / g 160
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com