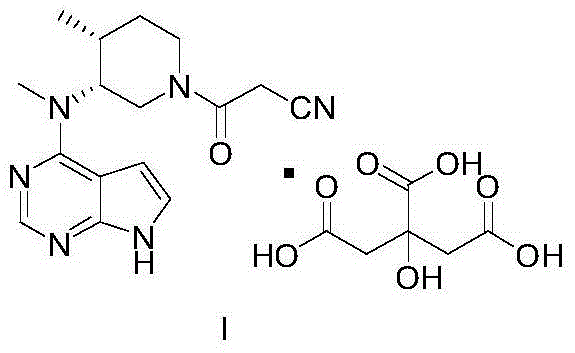
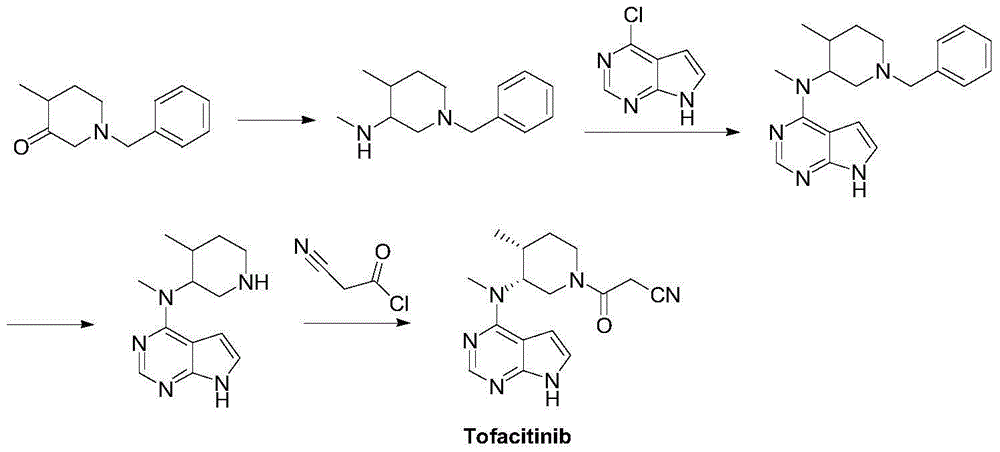
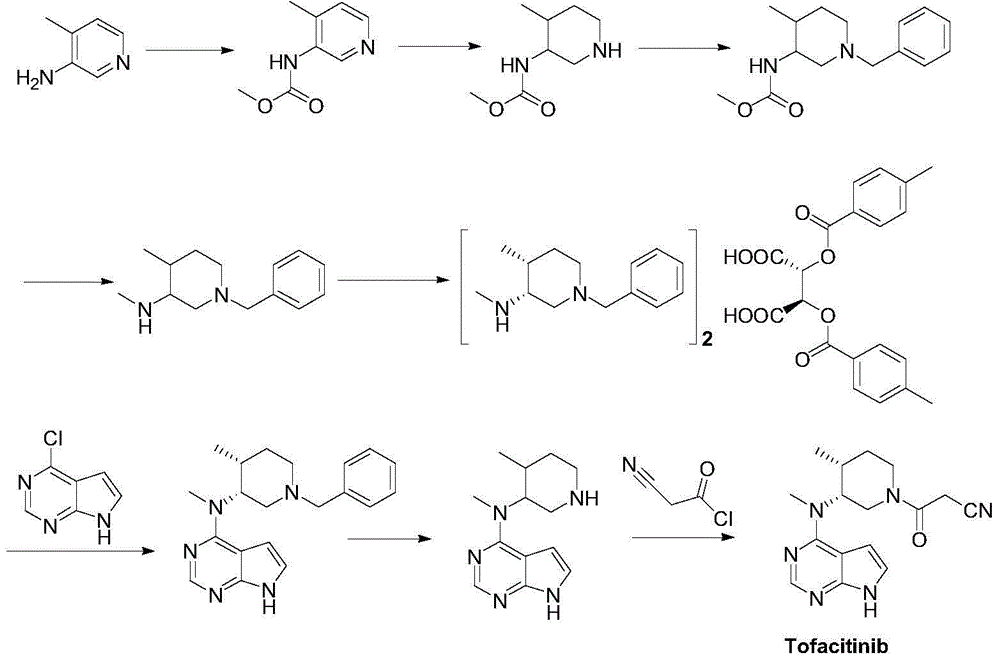
Preparation method of tofacitinib
A methyl, -4- technology, applied in the field of pharmaceutical preparation, can solve the problems of difficult control of enantiomeric impurity limit, expensive raw materials, long synthesis route, etc., and achieves easy operation and scale-up production, and the reaction route is simple and easy to operate and purify. The effect of the simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Synthesis of 1-benzyl-4-methyl-1,2,3,6-tetrahydropyridine (Ⅳ)
[0034] Benzyl bromide (17.1g, 100mmol) and pyridine (7.9g, 100mmol) were reacted at 20°C for 24h, the viscous liquid was heated to 130°C for 1h, and the reaction liquid was cooled to room temperature. Ethanol (120 mL) was added to dissolve, and the resulting yellow solution was cooled to 0° C., and sodium borohydride (5.0 g, 130 mmol) was added in portions within 2 h. The reaction solution was continued to react at 20°C for 12h. Water (60 mL) was slowly added dropwise to control the temperature of the system at 5-10° C., and diatomaceous earth (4.0 g) was added. Continue to stir at 0°C for 5 h, filter, wash the filter cake with ethanol (3×15 mL), combine the organic phases, concentrate under reduced pressure to a volume of about 50-60 mL, add dropwise sodium hydroxide solution (0.25M, 50 mL), methyl Extracted with tert-butyl ether (3×40 mL), combined the organic layers, washed with saturated brine (2×30 m...
Embodiment 2
[0036] Synthesis of (3R,4R)-4-methyl-1-(phenylmethyl)-3-piperidinol (V)
[0037] 1-Benzyl-4-methyl-1,2,3,6-tetrahydropyridine (18.7g, 100mmol) was dissolved in tetrahydrofuran (150mL), and sodium borohydride (6.5g, 170mmol ). The temperature of the reaction solution was lowered to 0°C, and a mixed solution of boron trifluoride diethyl ether (16.8g, 118.3mmol) and tetrahydrofuran (25mL) was slowly added dropwise to control the system temperature to ≤0°C. Return to room temperature and stir for 1.5h. The reaction solution was cooled to 0°C again, and water (50 mL) was slowly added dropwise to the system to destroy excess borane. After the reaction solution was stirred at room temperature for 2 h, it was lowered to 0°C, and a mixed solution of potassium hydrogen persulfate (110 g, 342.8 mmol) and water (500 mL) was slowly added dropwise. After the addition was complete, the system was warmed to room temperature for 12 h. The excess oxidant was quenched with sodium bisulfite in t...
Embodiment 3
[0039] N-methyl-N-((3R,4R)-4-methyl-1-phenylmethylpiperidin-3-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine ( VII) Synthesis
[0040] N-methyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine (14.8g, 100mmol) and (3R,4R)-4-methyl-1-(phenylmethyl)-3- Piperidinol (20.5g, 100mmol) was dissolved in dioxane (200mL), triphenylphosphine (26.2g, 100mmol) was added under stirring, the reaction solution was cooled to 0°C, diisopropyl azodicarboxylate was added dropwise (20.2g, 100mmol), after the dropwise addition, return to room temperature and react for 2h, then raise the temperature to 100°C and react for 2h, cool to room temperature, concentrate under reduced pressure to obtain a tan solid, add dichloromethane (200mL) to dissolve, and successively wash with saturated carbonic acid Sodium hydrogen (2×50 mL), washed with saturated brine (2×50 mL), and dried over anhydrous sodium sulfate. The solvent was concentrated under reduced pressure to obtain 22.8 g of anhydrous oily liquid with a yield of 68%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com