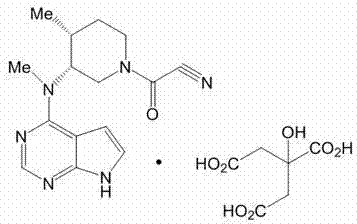
Tofacitinib tablets and preparation method thereof
A technology for tofacitinib and nigra tablets, which is applied in the field of tofacitinib tablets and their preparation, and achieves the effects of good stability, convenient medication and improved medication compliance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
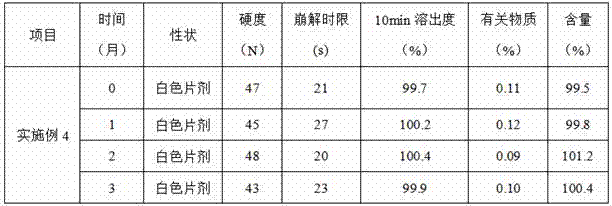
[0018] 5 parts of tofacitinib citrate, 75 parts of mannitol, and 10 parts of hydroxypropylmethylcellulose acetate succinate were passed through a 100-mesh sieve, and after mixing evenly, they were placed in a fluidized bed granulator, and Top spray operation mode, wet granulation. The obtained granules were dried under reduced pressure at 40°C, and then passed through a 60-mesh sieve for granulation. The above granules are mixed with 1 part of croscarmellose sodium, 3 parts of aspartame and 2 parts of magnesium stearate, and then compressed into tablets to obtain the preparation.
Embodiment 2
[0020] 10 parts of tofacitinib citrate, 50 parts of lactose, and 25 parts of methacrylic acid copolymer S were passed through a 100-mesh sieve, mixed evenly, and placed in a wet granulator for wet granulation. The obtained granules were dried under reduced pressure at 40°C, and then passed through a 60-mesh sieve for granulation. The above-mentioned granules are mixed with 10 parts of crospovidone, 3 parts of aspartame and 2 parts of magnesium stearate, and then compressed into tablets to prepare.
Embodiment 3
[0022] 10 parts of tofacitinib citrate, 55 parts of lactose, 20 parts of mannitol, and 5 parts of ethyl cellulose were passed through a 100-mesh sieve, mixed evenly, and then granulated by a dry granulator. The resulting granules are sieved through a 60-mesh sieve. The above-mentioned granules are mixed with 5 parts of croscarmellose sodium, 1 part of aspartame and 4 parts of magnesium stearate, and then compressed into tablets to prepare.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com