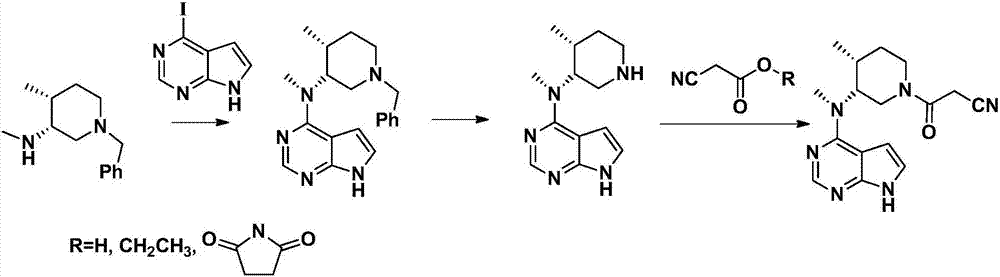
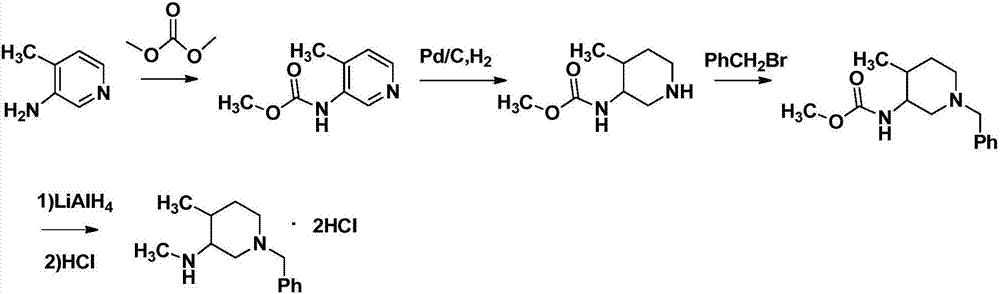
Preparation method of tofacitinib intermediate
A technology for tofacitinib and intermediates, which is applied in the field of preparation of tofacitinib intermediates, can solve problems such as unfavorable industrial production, difficulty in separation and purification, low yield, etc. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
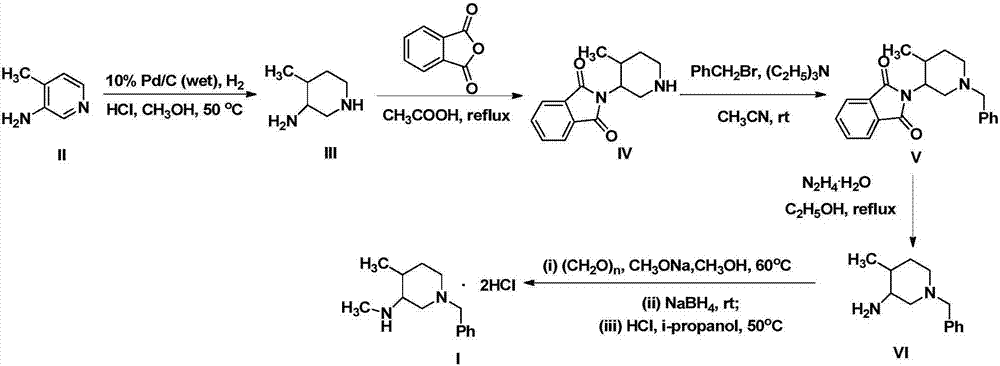
[0061] Preparation Example 1: Preparation of Compound III
[0062]
[0063] Set up experimental groups 1 to 5 to prepare compound III respectively. The method is as follows: put compound II, acid, 100mL solvent, and 10% Pd / C (wet) in a hydrogenation kettle at 45 to 55°C under a hydrogen pressure of 4 to 8 MPa React for 16-24 hours until no hydrogen is absorbed, and TLC checks that the reaction of the raw materials is complete. Cool to room temperature, filter, and concentrate the filtrate under reduced pressure, add ethyl acetate (50 mL) to dissolve, adjust the pH to 12 with 5% aqueous sodium hydroxide solution, separate the layers, and extract the aqueous layer twice with ethyl acetate (30 mL×2) , combined organic phases, dried over anhydrous sodium sulfate for 6 h, filtered, and concentrated under reduced pressure to obtain compound III. The differences and experimental results of experimental groups 1 to 5 are shown in Table 1 below:
[0064] Table 1
[0065]
[00...
preparation Embodiment 2
[0067] Preparation Example 2: Preparation of Compound IV
[0068]
[0069] Set up experimental groups 1 to 6 to prepare compound III respectively. The method is as follows: add compound III, phthalic anhydride, and 40 mL of solvent to the reaction flask, and react at 60 to 150° C. for 1 to 12 hours under stirring conditions, and reduce to At room temperature, add 40 mL of ethanol solution saturated with hydrogen chloride, stir for 1 h until the solid precipitates, continue to stir for 5 h, stand at 0°C overnight until the solid precipitates completely, filter, and add the filter cake to the mixed solution of dichloromethane (50 mL) and water (20 mL) , the pH was adjusted to 9-10 with saturated aqueous sodium carbonate solution, the organic phase was extracted and collected, the aqueous phase was extracted twice with dichloromethane (50 mL×2), dried over anhydrous sodium sulfate for 6 h, filtered, and concentrated under reduced pressure to obtain compound IV. The differences...
preparation Embodiment 3
[0073] Preparation Example 3: Preparation of Compound V
[0074]
[0075] Set up experimental groups 1 to 6 to prepare compound V respectively. The method is as follows: add compound IV, 60mL solvent, and alkali to the reaction flask, add the solution (25mL) of the aforementioned solvent of benzyl bromide dropwise under ice bath, and rise to room temperature after dropping React at ~80°C for 2 hours, concentrate under reduced pressure and distill off the solvent, add water (50mL) and dichloromethane (100mL), stir for 10min, separate layers, wash the dichloromethane layer twice with saturated brine (50mL×2), organic The phase was dried with anhydrous sodium sulfate for 6 h, filtered, and concentrated under reduced pressure to obtain compound V. The differences and experimental results of experimental groups 1 to 6 are shown in Table 3 below:
[0076] table 3
[0077]
[0078] Characterization data of Compound V: 1 H-NMR(DMSO,300MHz)δ:1.02(m,3H,C H 3 CH); 1.25~1.29(m,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com