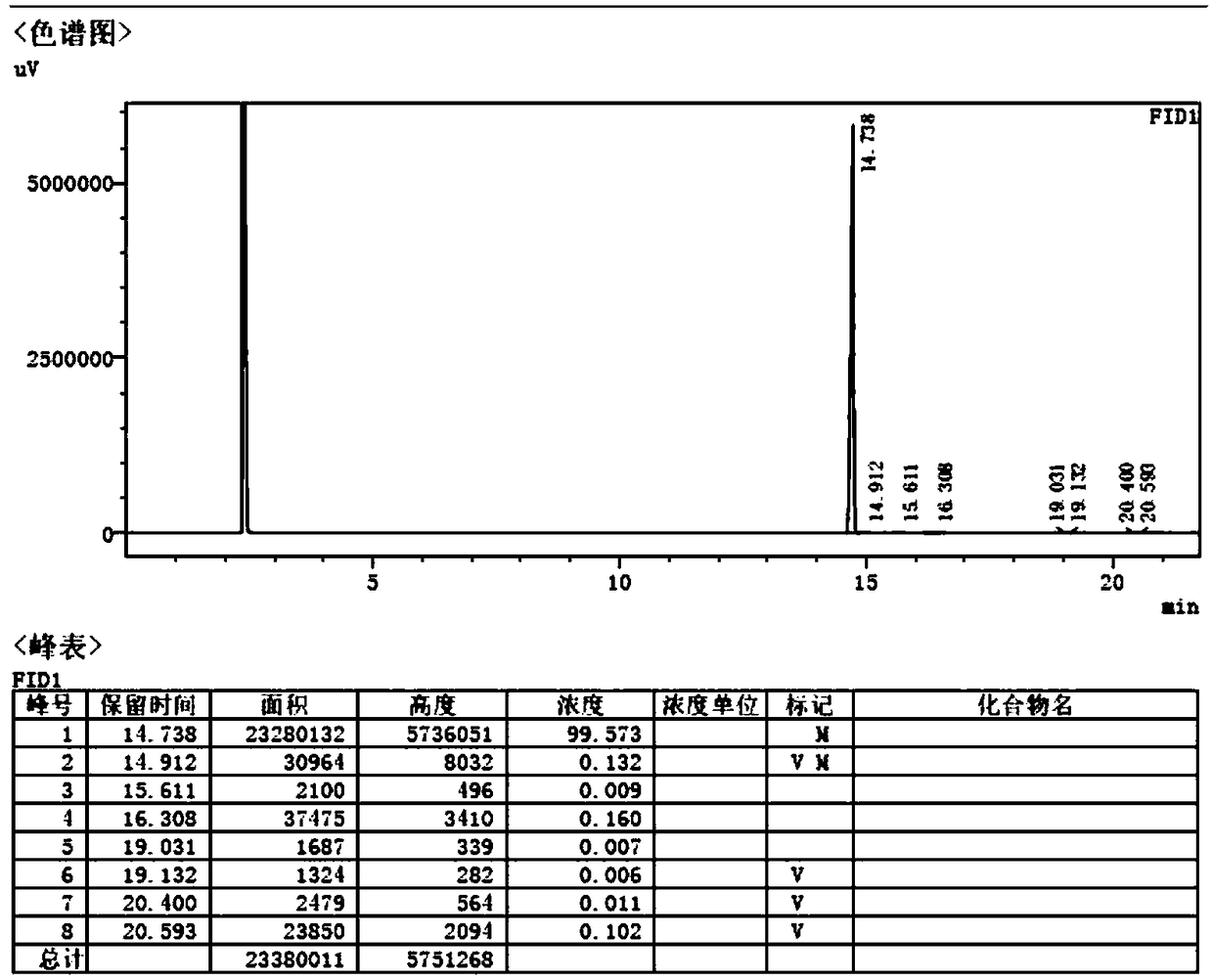
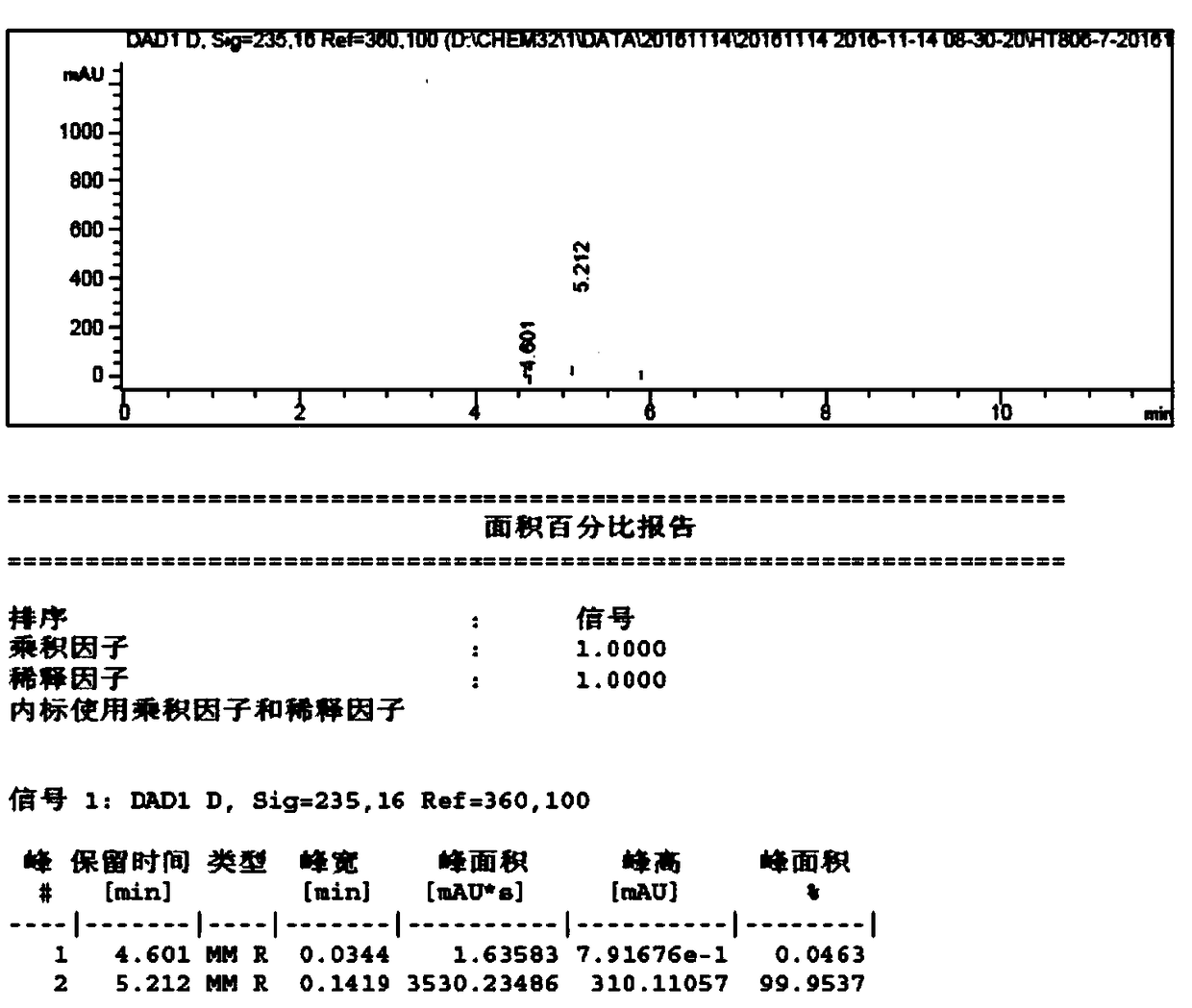
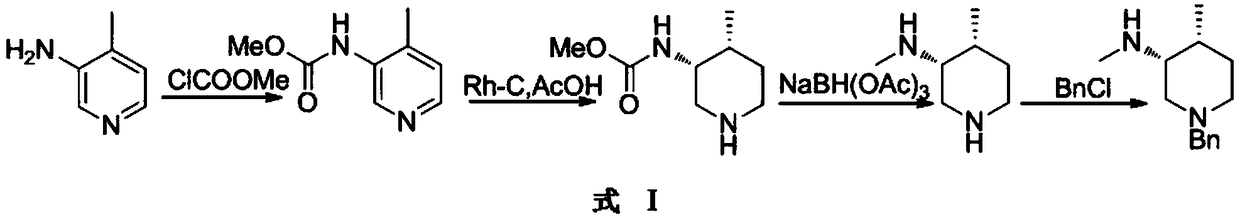
Synthesis method for preparing intermediates of tofacitinib
A technology of tofacitinib and intermediates, applied in the field of synthesis of organic compounds, can solve problems such as danger and difficulty in scale-up production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment one: X is a chlorine atom, X 1 is a bromine atom.
[0040]
[0041] Take 10 moles of the compound 3-bromo-4-methylpyridine and dissolve it in an aqueous solution of methylamine containing 40 moles of methylamine, then add 0.01 moles of copper powder to it, and then heat it to 150-160 degrees to react. After the raw materials disappear, stop heating , cooled to room temperature, the reaction liquid was extracted with dichloromethane, and then the organic phase was concentrated to obtain product 4 (9.5 moles), and the reaction yield was 95%.
[0042] The obtained compound 4 was dissolved in 10 times the volume of methyl tert-butyl ether, and then 19 moles of benzyl chloride was added thereto, and stirred until the reaction was complete to obtain a suspension of compound 5. Slowly add the suspension to a solution of 10 times the volume of ethanol containing 38 moles of sodium borohydride between 60 and 70 degrees. After the addition is complete, continue to ...
Embodiment 2
[0044] Embodiment two: X is a bromine atom, X 1 for the chlorine atom.
[0045]
[0046] Take 30 moles of the compound 3-chloro-4-methylpyridine and dissolve it in an aqueous solution containing 1.2 times the molar amount of methylamine, then add 0.2 moles of cuprous chloride to it, and then heat it to 140-150 degrees to react. After the raw materials disappear , stop heating, cool to room temperature, extract the reaction liquid with dichloromethane, then concentrate the organic phase to obtain product 4, and the reaction yield is 65%.
[0047] The obtained compound 4 was dissolved in 5 times the volume of dichloromethane, and then 21 moles of benzyl bromide was added dropwise thereto, and stirred until the reaction was complete to obtain a suspension of compound 5. Slowly add the suspension into an ethanol solution containing 2 times the molar amount of sodium borohydride and 10 times the volume of ethanol solution between 60-70 degrees. The reaction was quenched, the r...
Embodiment 3
[0049] Embodiment three: X is a chlorine atom, X 1 is a bromine atom.
[0050]
[0051] Take 20 moles of the compound 3-bromo-4-methylpyridine and dissolve it in an aqueous solution containing 2.0 times the molar amount of methylamine, then add 0.1 moles of copper powder to it, then heat the reaction (between 90-100 degrees), and the reaction is completed Afterwards, heating was stopped, cooled to room temperature, and the reaction solution was directly filtered to remove solids, then concentrated under reduced pressure and evaporated to dryness to obtain product 4, with a reaction yield of 70%.
[0052] Dissolve the obtained compound 4 in 2 volumes of acetone, then dropwise add 17 moles of benzyl chloride therein, stir until the reaction is complete, solid precipitates, cool and filter to obtain compound 5; then the solid compound 5 obtained above is redissolved in ethanol , the resulting solution is slowly added dropwise to an aqueous ethanol solution containing 40 moles...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com