Crystalline and non-crystalline forms of tofacitinib, and a pharmaceutical composition comprising tofacitinib and a penetration enhancer
A composition and form technology for drug combination, drug delivery, non-central analgesics, etc., capable of solving problems such as unpredictable, daunting results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
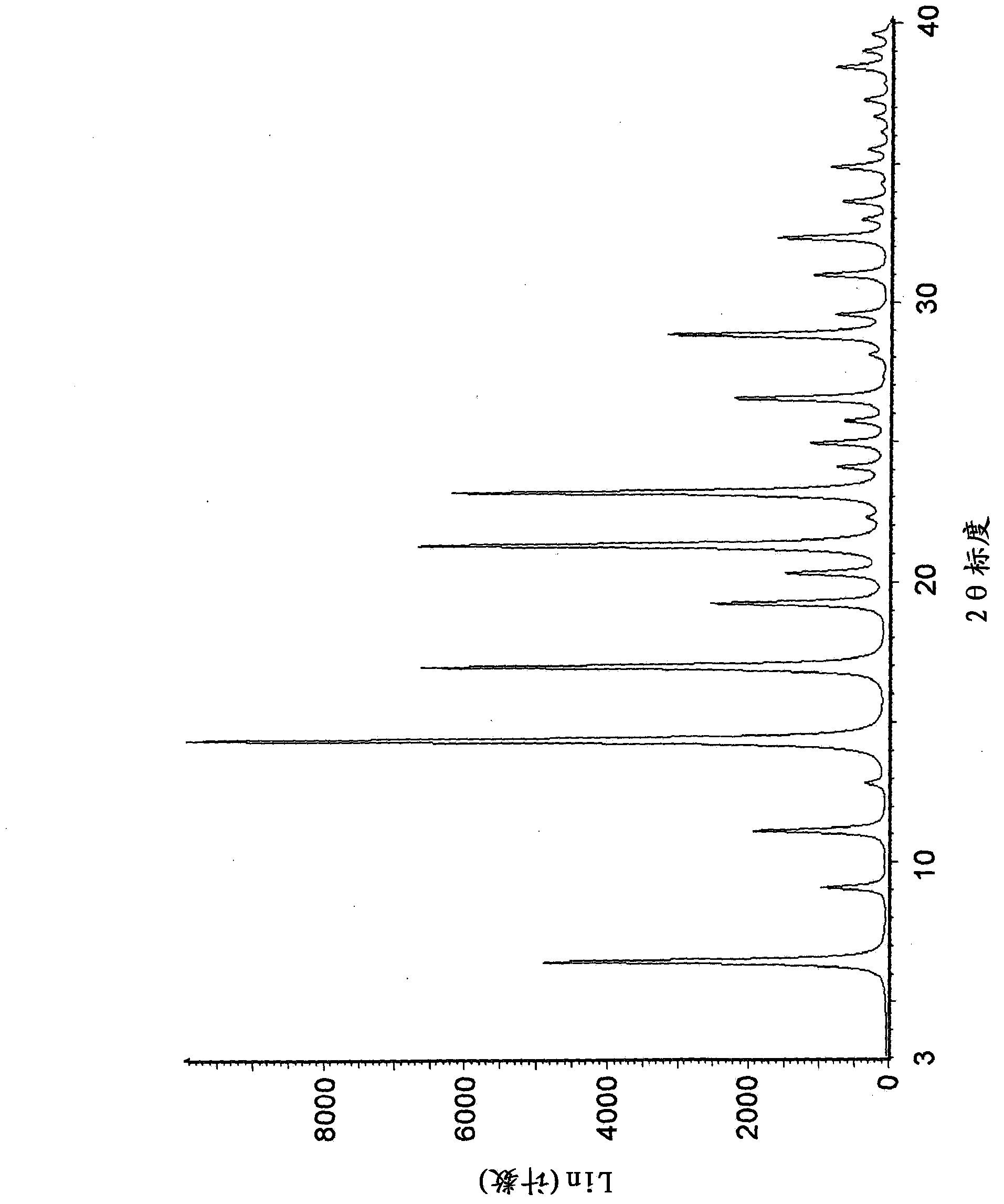
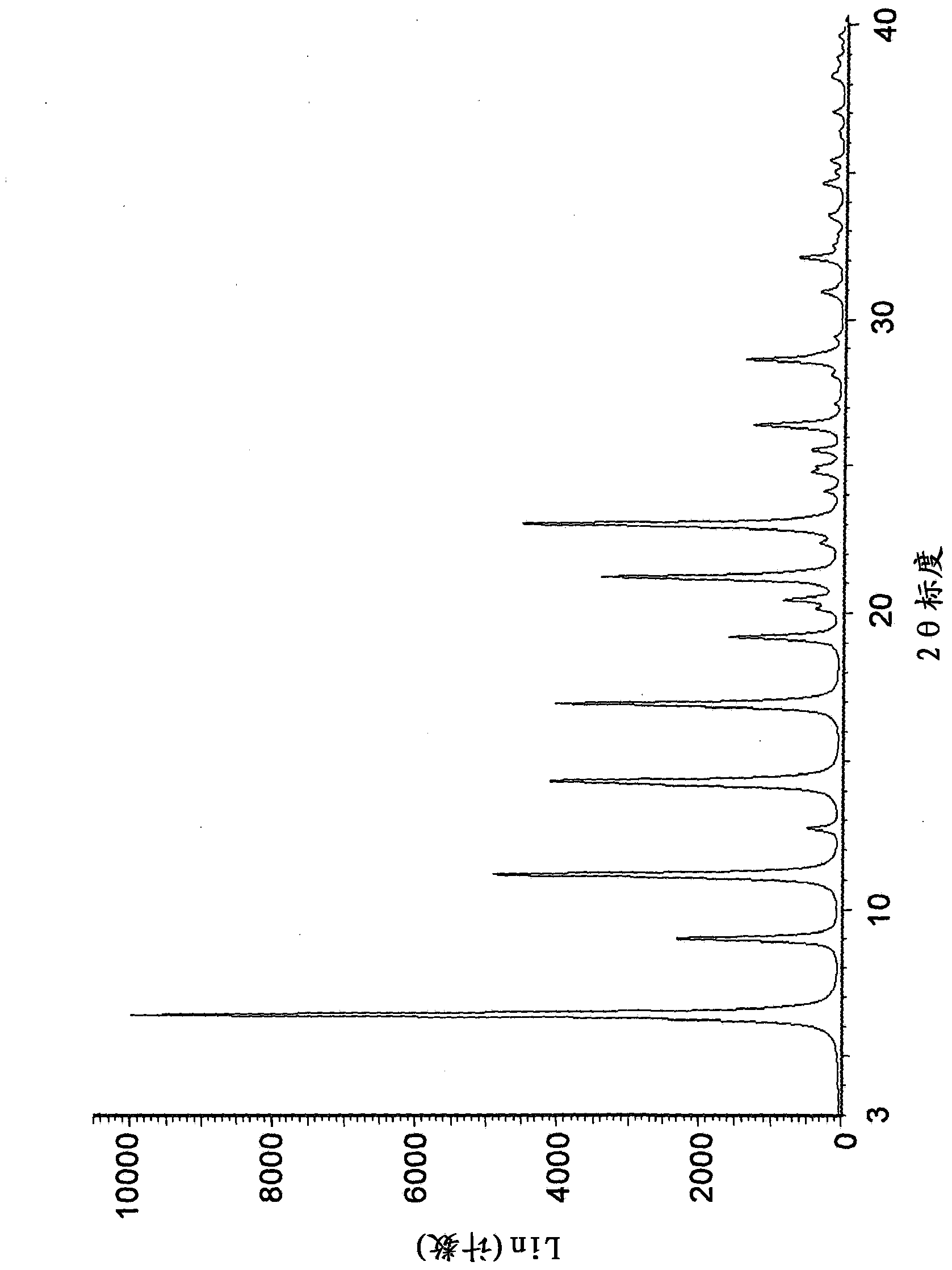
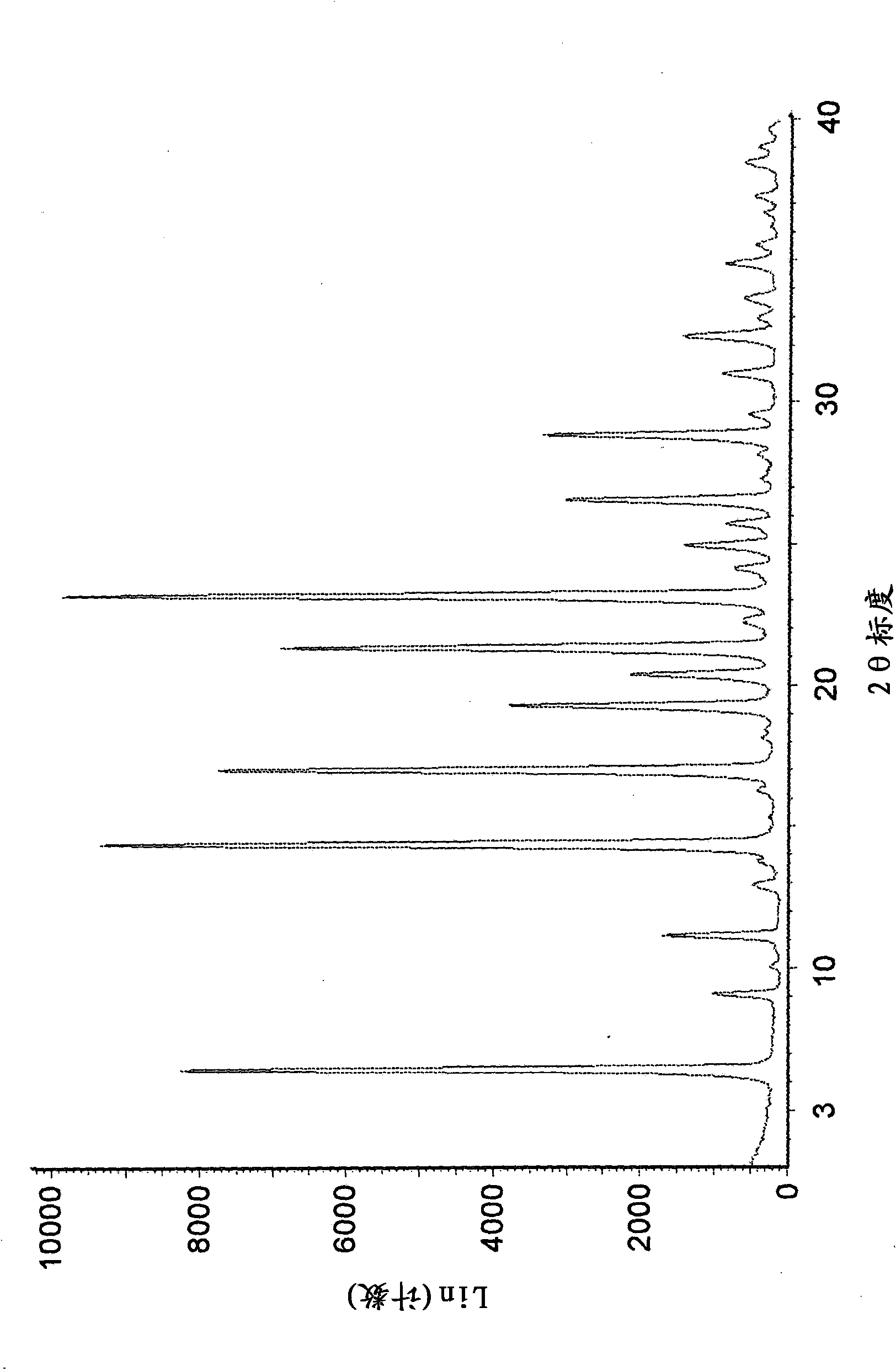
[0242] 2-propanolate (method 1): by adding 750 g of 3-((3R,4R)-4-methyl-3-[methyl- (7H-Pyrrolo[2,3-d]pyrimidin-4-yl)-amino]-piperidin-1-yl)-3-oxopropionitrile citrate to prepare a crystalline form. The resulting mixture was stirred at 20°C for about 1 hour. Then 4 L of 1M aqueous sodium hydroxide solution was added to the mixture over 40 minutes. The mixture was then stirred at 20°C for about 17 hours. The solid was isolated by vacuum filtration, washed twice with 1.9 L of water, and dried under reduced pressure at 65°C for about 30 hours. The resulting crystalline solid contained 1.0 wt% water by Karl-Fischer analysis and 2.6 wt% 2-propanol by residual solvent analysis.
Embodiment 2
[0244] 2-propanolate (method 2): 271 g of 3-((3R,4R) -4-Methyl-3-[methyl-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-amino]-piperidin-1-yl)-3-oxopropionitrile citric acid salts to prepare crystalline forms. Mixing was facilitated with an overhead stirrer throughout the experiment. While providing high speed agitation to the slurry, 1.88 L of 1.0 N aqueous sodium hydroxide solution was slowly added at 20°C. The reactor was then seeded with 1 wt% crystalline form and stirred at ambient temperature for several hours to obtain a slurry. The solid was isolated by vacuum filtration, washed with water and dried under reduced pressure at 60-70°C. The resulting crystalline solid contained 0.9% by weight water and 2.8% by weight 2-propanol as determined by Karl-Fischer analysis and residual solvent analysis, respectively.
Embodiment 3
[0246]2-propanolate (method 3): by adding 218 mg of non-crystalline 3-((3R,4R)-4-methyl-3-[methyl-(7H-pyrrolo[2, 3-d]pyrimidin-4-yl)-amino]-piperidin-1-yl)-3-oxopropionitrile to prepare a crystalline form. The mixture was stirred at room temperature for about 5 days, isolated by vacuum filtration and dried under reduced pressure at 70 °C for 1 day. The resulting crystalline solid contained 4.7% by weight 2-propanol by residual solvent analysis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com