Synthesis method of JAK inhibitor tofacitinib
A technology for the synthesis of tofacitinib and its synthesis method, which is applied in the field of synthesis of the JAK inhibitor tofacitinib, can solve the problems of low yield and long reaction time, and achieve the effects of simple operation, easy control of impurities, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
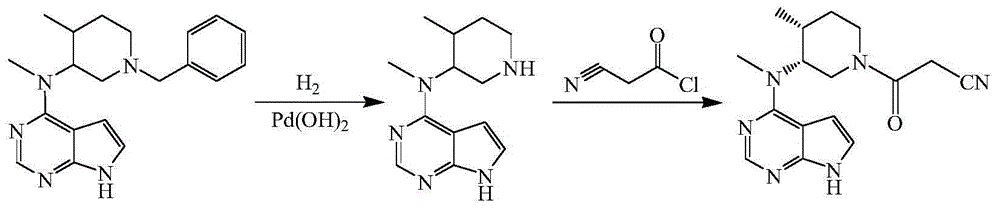
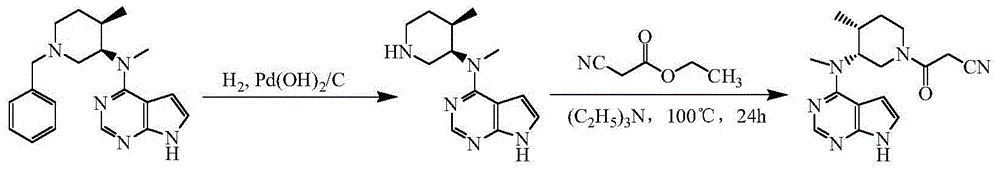
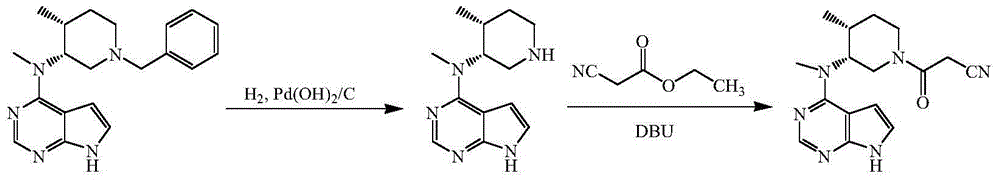
[0033] 5% palladium on carbon (1g, 59% water content), N-[(3R,4R)-1-benzyl-4-methylpiperidin-3-yl]-N-methyl-7H-pyrrolo[2, 3-d] Pyrimidin-4-amine (6.7g, 0.02mol), ethanol (50mL), 3mL of formic acid were added to a 100mL three-necked flask, protected by nitrogen, heated to 40°C and reacted for 12h to complete the reaction, adding sodium carbonate to adjust the pH of the system to 7-8, remove the solid by filtration, and evaporate the filtrate to remove ethanol under reduced pressure to obtain N-[(3R,4R)-4-methylpiperidin-3-yl]-N-methyl-7H-pyrrolo[2, 3-d] Pyrimidin-4-amine 4.0 g, yield 81.6%.
[0034] N-[(3R,4R)-4-methylpiperidin-3-yl]-N-methyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine (2.5g, 0.01mol), Cyanoacetic acid (1.0 g, 0.012 mol), DCC (4.1 g, 0.02 mol), DMAP (0.05 g), tetrahydrofuran (25 mL). Add it into a 50mL three-necked flask, raise the temperature to 35°C and react for 1h, adjust the pH to 7-8 with sodium carbonate, remove the solid by filtration, concentrate the filtrate...
Embodiment 2
[0036] 20% palladium hydroxide on carbon (0.5g, 50% water), N-[(3R,4R)-1-benzyl-4-methylpiperidin-3-yl]-N-methyl-7H-pyrrolo [2,3-d]Pyrimidin-4-amine (6.7g, 0.02mol), methanol (50mL), ammonium formate (3.15g, 0.05mol), add to a 100mL three-necked flask, protect with nitrogen, heat up to 60°C for reaction After 6h, the reaction was complete, adding sodium carbonate, adjusting the pH of the system to 7-8, filtering to remove the solid, and distilling the filtrate to remove methanol under reduced pressure to obtain N-[(3R,4R)-4-methylpiperidin-3-yl]-N -Methyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine 4.1 g, yield 93.3%.
[0037] N-[(3R,4R)-4-methylpiperidin-3-yl]-N-methyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine (2.5g, 0.01mol), Cyanoacetic acid (1.0 g, 0.012 mol), DIC (2.5 g, 0.02 mol), 4-PPY (0.05 g), dichloromethane (25 mL). Add it into a 50mL three-necked flask, raise the temperature to 35°C and react for 1h, adjust the pH to 7-8 with potassium carbonate, remove the solid by filtration, ...
Embodiment 3
[0039] 5% palladium on carbon (1.0g, 59% water content), N-[(3R,4R)-1-benzyl-4-methylpiperidin-3-yl]-N-methyl-7H-pyrrolo[2 ,3-d] Pyrimidin-4-amine (6.7g, 0.02mol), ethanol (50mL), hydrazine hydrate (80%, 4g), added to a 100mL three-necked flask, protected by nitrogen, heated to 75°C for 4h and the reaction was complete , filtered to remove the solid, the filtrate was concentrated, and column chromatography was separated to obtain N-[(3R,4R)-4-methylpiperidin-3-yl]-N-methyl-7H-pyrrolo[2,3-d] Pyrimidin-4-amine 3.6g, yield 73.5%.
[0040] N-[(3R,4R)-4-methylpiperidin-3-yl]-N-methyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine (2.5g, 0.01mol), Cyanoacetic acid (1.0 g, 0.012 mol), DCC (4.1 g, 0.02 mol), HOSU (0.05 g), tetrahydrofuran (25 mL). Add it into a 50mL three-necked flask, raise the temperature to 50°C and react for 1h, adjust the pH to 7-8 with sodium bicarbonate, remove the solid by filtration, concentrate the filtrate, and separate by column chromatography to obtain 2.5g of tofa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com