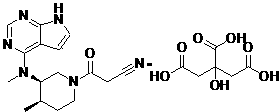
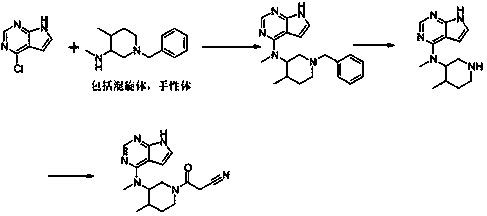
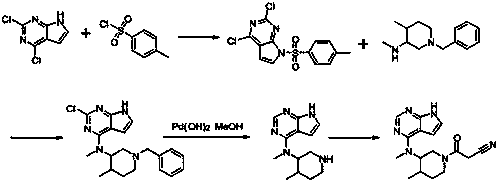
Method for preparing tofacitinib
A technology of tofacitinib and its compound, which is applied in the field of preparation of tofacitinib, can solve the problems of hindering the domestic industrial production of tofacitinib citrate, unfavorable industrial production, and many by-products formed, so as to achieve low production cost, Ease of purification and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The following examples help to understand the present invention, but do not limit the content of the present invention.
[0031] Example 1: 3-{(3R,4R)-4-methyl-3-[methyl-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino] with the structural formula shown in II Preparation of ethyl piperidin-1-yl}-3-oxopropionate
[0032] Add 240ml of anhydrous methanol, 24.5g of compound Ⅰ (0.1mol), 17.62g of diethyl malonate (0.11mol) and 11.1g of triethylamine (0.11mol) into a 500ml four-neck flask, stir and reflux for 9h, thin layer After the reaction was analyzed, methanol was removed under reduced pressure, 50 ml of water was added, extracted with ethyl acetate (120 ml*2), the organic layer was washed with saturated sodium chloride, dried over anhydrous sodium sulfate, filtered, and precipitated under reduced pressure to obtain 30.5 g of compound II. The yield is 85%.
[0033] MS: m / z 360.1 (MH + ); 1 HNMR(600 MHz)(CDCl3):1.05-1.10(3H,m), 1.22-1.31(1H,m), 1.69-1.72(1H,m), 1.87-1.91(1H,m),...
Embodiment 2
[0034] Example 2: 3-{(3R,4R)-4-methyl-3-[methyl-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino] with structural formula III Preparation of piperidin-1-yl}-3-oxopropionamide
[0035] Into a 500ml four-neck flask, add 200ml methanol, 17.97g compound II (0.05mol) and 18.8g 28% ammonia water (0.15mol) in sequence, stir and reflux for 3 hours, after the reaction is completed by TLC analysis, the solvent is removed under reduced pressure, and 50ml of water is added. Extract with methyl chloride (150ml*2), combine the dichloromethane layers, wash with saturated sodium chloride, dry over anhydrous sodium sulfate, filter, and concentrate under reduced pressure to obtain 14.86g of compound III with a yield of 90%.
[0036] MS: m / z331 (MH + ); 1 HNMR(600MHz)(DMSO):0.99-1.02(3H,d), 1.53-1.82(2H,d), 2.35-2.41(1H,m), 3.16-3.17(1H,d), 3.27-.334(5H ,m), 3.49-3.51(1H,s), 3.66-3.91(2H,m), 5.76(1H,d), 6.55(1H,s), 6.95-7.02(1H,d), 7.13(1H,t ), 7.41-7.48(1H,d), 8.11(1H,m), 11.62-11.64(1H,m).
Embodiment 3
[0037]Example 3: 3-{(3R,4R)-4-methyl-3-[methyl-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino] with structural formula III Preparation of piperidin-1-yl}-3-oxopropionamide
[0038] Add 120ml of anhydrous methanol, 12.25g of compound I (0.05mol), 13.1g (0.1mol) of ethyl 3-amino-3-oxopropionate and 11.4g of DBU (0.075mol) into a 500ml four-necked flask, and stir and reflux for 12h , evaporated the solvent under reduced pressure, added 30ml of water to the residue, extracted with dichloromethane (100ml*2), collected the dichloromethane layer, washed with saturated sodium chloride, dried over anhydrous sodium sulfate, filtered, and precipitated under reduced pressure to obtain The residue was purified by dichloromethane / anhydrous methanol (10:1) column chromatography to obtain compound III.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com