Industrial production method of citric acid tofacitinib
A production method and technology of tofacitinib, applied in the directions of organic chemistry, carboxylate preparation, drug combination, etc., can solve the problems of unsuitability for industrial production, difficulty in ensuring product quality, and few active ester suppliers, etc. The effect of industrialized production, low production cost and short time consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
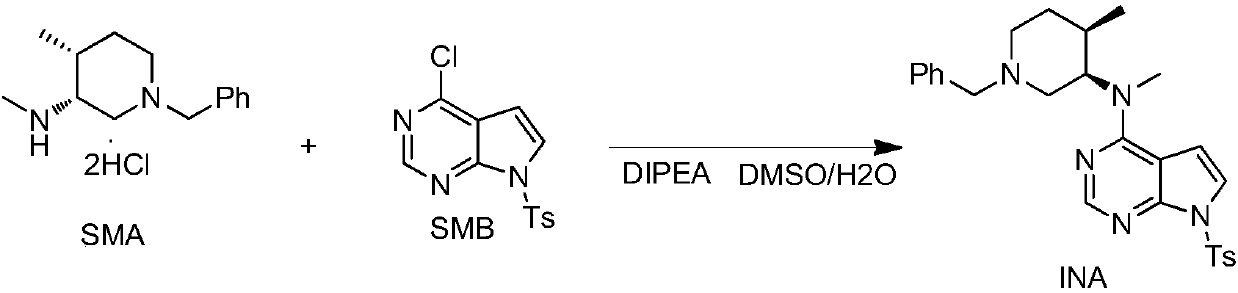
[0043] The specific synthesis operation of the intermediate INA is as follows:
[0044] Example 1a: Add 11.0kg of SMA, 12.8kg of SMB, 19.5kg of DIPEA, 50.0kg of DMSO, and 15.0kg of purified water into a 200L reactor, and raise the temperature to 107°C for reaction. After the reaction is complete, cool down to room temperature and add 24.0 kg of absolute ethanol and 30.0 kg of purified water, stir and crystallize for 2 hours, filter with suction, and dry at 60±5°C to obtain 17.2 kg of off-white solid. Yield 93.0%, SMB remaining 0.3%, HPLC purity 98.9%.
[0045] Example 1b: Add 11.0kg of SMA, 12.8kg of SMB, 19.5kg of DIPEA, 48.0kg of DMSO, and 12.0kg of purified water into a 200L reactor, and raise the temperature to 112°C for reaction. After the reaction is complete, cool down to room temperature and add 24.0 kg of absolute ethanol and 30.0 kg of purified water, stir and crystallize for 2 hours, filter with suction, and dry at 60±5°C to obtain 16.9 kg of off-white solid. Yiel...
Embodiment 2
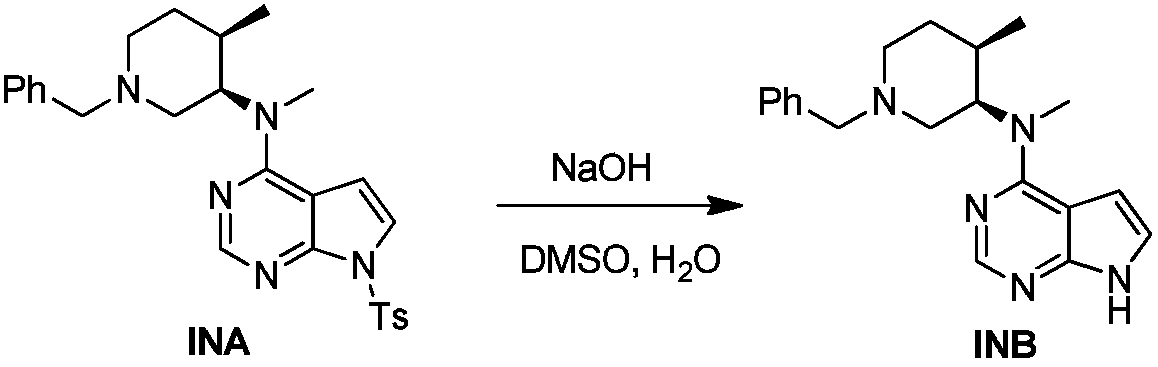
[0051] The specific synthesis operation of intermediate INB is as follows:
[0052] Example 2a: 15kg of the intermediate INA prepared in Example 1c, 65kg of DMSO, 15kg of purified water, and 6.15kg of sodium hydroxide were added to a 100L reactor, and the temperature was raised to 80°C for reaction. After the reaction is complete, cool down to room temperature, stir and crystallize for 2 hours, shake off the filter, and dry at 60±5°C to obtain 9.8kg of yellow solid.
[0053] Example 2b: Put 2.0kg of the dried sample obtained in Example 2a into a 20L reaction kettle, add 12kg of absolute ethanol, heat to 63°C until the solid is completely dissolved, add 100g of medicinal charcoal for decolorization for 15min, suction filter while it is hot, and transfer the filtrate to 20L In the reaction kettle, cool down to 0-30°C, stir and crystallize for 2h, filter with suction, and dry at 60±5°C to obtain 1.80kg of yellow solid. The total yield was 85.8%, the intermediate INA was not dete...
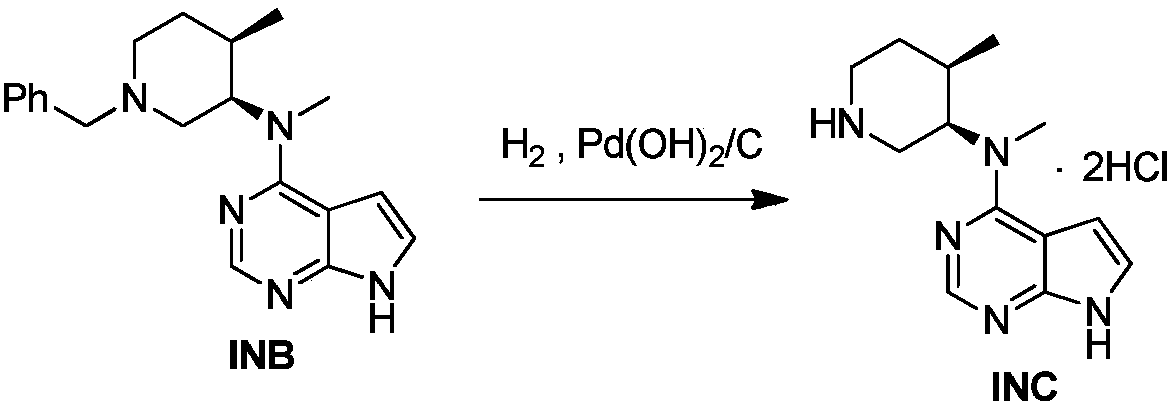
example 2
[0062] Example 2g: 15kg of the intermediate INA prepared in Example 1c, 60kg of DMSO, 30kg of purified water, and 6.15kg of sodium hydroxide were added to a 100L reactor, and the temperature was raised to 65-95°C for reaction. After the reaction is complete, cool down to room temperature, stir and crystallize for 2 hours, shake off filter, and dry at 60±5°C to obtain 9.6kg of yellow solid.
[0063] Place the dried sample in a 100L reaction kettle, add 57.6kg of absolute ethanol, heat to 64°C until the solids are completely dissolved, add 480g of medicinal charcoal for decolorization for 15min, suction filter while it is hot, transfer the filtrate to a 200L reaction kettle, and cool down to Stir and crystallize at 0-30°C for 2h, filter with suction, and dry at 60±5°C to obtain 8.5kg of yellow solid. The total yield was 82.7%, the intermediate INA was not detected, and the HPLC purity was 100.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com