Tofacitinib intermediate and preparation method thereof
A solid and compound technology, which is applied in the preparation of carboxylate, organic chemistry methods, organic chemistry, etc., can solve the problems of poor stability, easy to be oxidized, and increase the difficulty of quality control of the compound of formula I, and achieve high yield and easy operation. Simple, Gentle Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Preparation of methyl-((3R,4R)-4-methyl-piperidin-3-yl)-(7H-pyrido[2,3-d]pyrimidin-4-yl)-amine (formula Ⅲ)
[0088] Method 1: the method for preparing formula III from formula IV
[0089] (3R,4R)-(1-Benzyl-4-methyl-piperidin-3-yl)-methyl-(7H-pyrido[2,3-d]pyrimidin-4-yl)-amine (formula Ⅳ) For the preparation method, refer to CN1729192. At room temperature, 8 g of (3R,4R)-(1-benzyl-4-methyl-piperidin-3-yl)-methyl-(7H-pyrido[2,3-d]pyrimidin-4-yl )-amine was added to a 200ml hydrogenation reactor, and 32ml of ethanol and 8ml of water were added to the reactor. Weigh 1.43g of acetic acid into the solution, stir, and the solids are completely dissolved. Weigh 1.6g of 20% palladium hydroxide on carbon and add it. The gas in the hydrogenation tank was replaced by nitrogen and hydrogen successively, then hydrogen was added to 0.7MPa, and then heated to an internal temperature of 50°C with stirring. After reacting for 5 hours, deflate and dismantle the device. After the reac...
Embodiment 2
[0093] Methyl-((3R,4R)-4-methyl-piperidin-3-yl)-(7H-pyrido[2,3-d]pyrimidin-4-yl)-amine.dihydrochloride (formula Ⅵ) Preparation
[0094] Method A:
[0095]5.0g of methyl-((3R,4R)-4-methyl-piperidin-3-yl)-(7H-pyrido[2,3-d]pyrimidin-4-yl)-amine was dissolved in 20ml of methanol , stir. At room temperature, a solution of 6ml of concentrated hydrochloric acid dissolved in 40ml of ethanol was added dropwise to the above solution, and crystals were precipitated. After dropping, stirred for 5 hours, filtered with suction, the filter cake was washed with 10ml of ethanol, and dried under reduced pressure at 50°C to constant weight to obtain off-white Solid title product (5.8 g, 85% yield, 99.2% purity by HPLC).
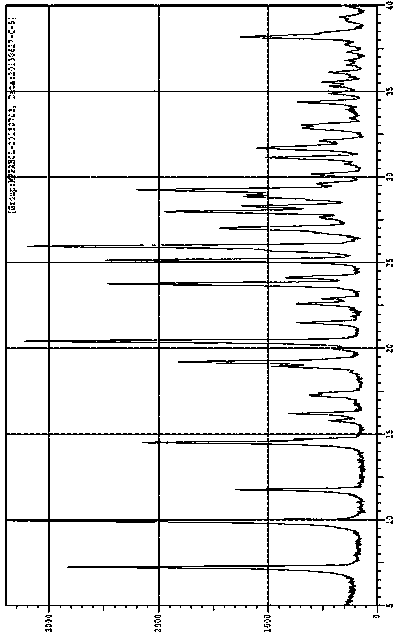
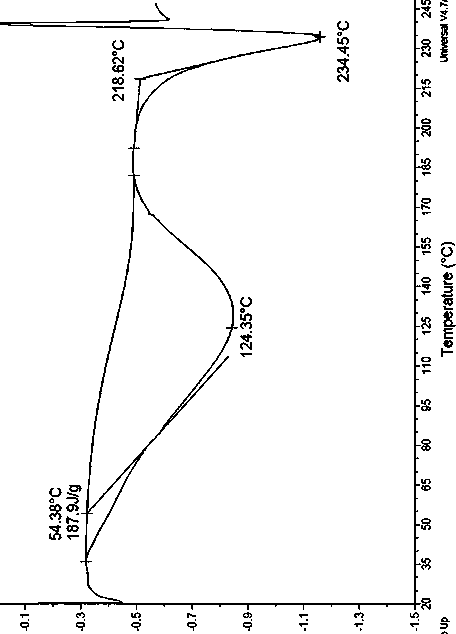
[0096] Determine the X-ray powder diffraction pattern (XRPD) of the obtained solid, the results are shown in figure 1 , which is Form A. The measured X-ray powder diffraction pattern, its 2θ angle, d value and relative intensity I value are shown in Table 3 below, and the ...
Embodiment 3
[0103] Preparation of Tofitinib Citrate
[0104] 5g methyl-((3R,4R)-4-methyl-piperidin-3-yl)-(7H-pyrido[2,3-d]pyrimidin-4-yl)-amine. dihydrochloride Suspend in 25ml of dichloromethane, adjust the pH of the solution to about 8.0 with 2mol / L sodium hydroxide aqueous solution, extract and separate the system, extract the water phase twice with 50ml of dichloromethane, combine the dichloromethane phase, and use 30ml of saturated saline After washing, dry the dichloromethane phase with 1.0 g of anhydrous sodium sulfate for 2 h. After filtration, the filtrate was concentrated to dryness under reduced pressure to obtain a foamy solid. The solid was added into a 100ml three-neck flask, 15ml of n-butanol, 3.3g of ethyl cyanoacetate, and 2.2g of DBU were added in sequence, stirred, protected by nitrogen, heated to an internal temperature of 40°C, and the reaction solution was stirred. Weigh 5.6g of citric acid and dissolve it in 30ml of acetone to prepare a solution. Thin chromatogra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com