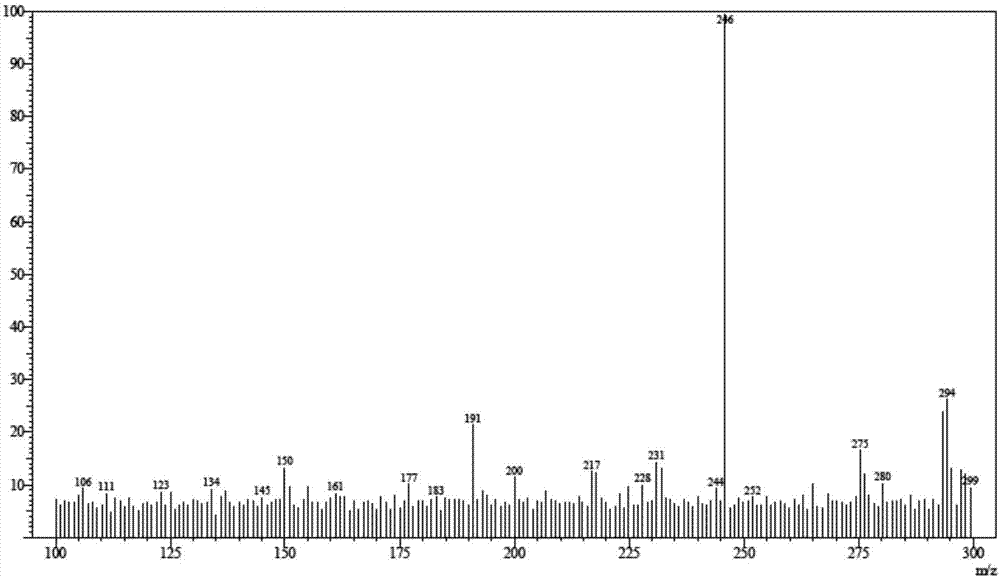
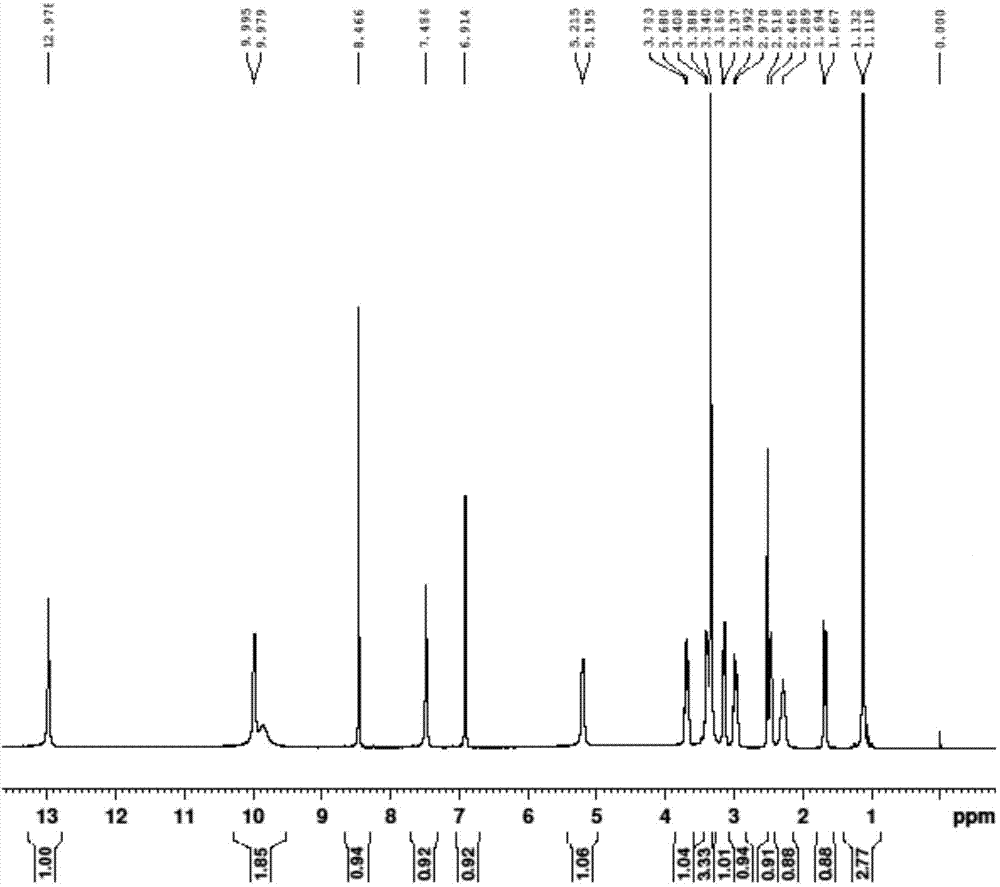
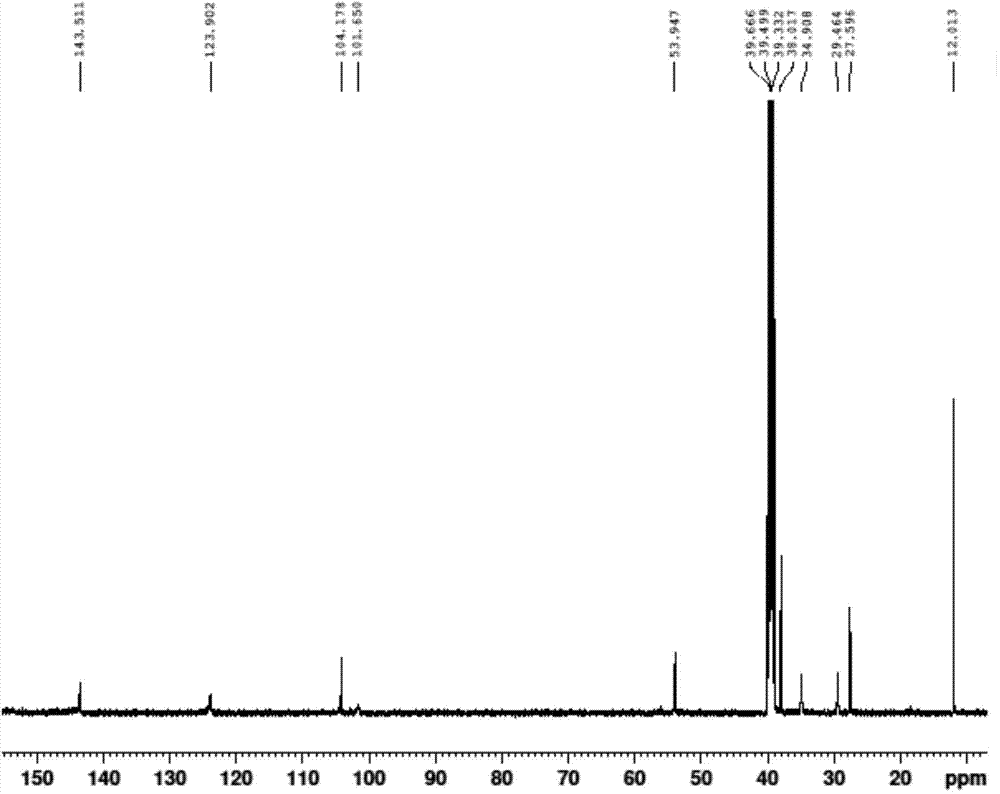
Tofacitinib intermediate preparation method and method for preparing tofacitinib or its salt by using tofacitinib intermediate preparation method
A technology for tofacitinib and medicinal salts is applied in the field of pharmaceutical synthesis, which can solve the problems of low product purity and difficult evaporation of water, and achieve the effects of reducing production costs, reducing the introduction of impurities, and overcoming the complexity of the preparation process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Preparation of N-methyl-N-((3R,4R)-4-methylpiperidin-3-yl)-7H-pyrrolo[2,3-D]pyrimidin-4-amine (compound of formula I)
[0041]
[0042] Add 35 g of compound II, 175 ml of acetonitrile, and 142 ml of hydrochloric acid (1 mol / L, relative molar weight is 1.5) into a dry 500 ml hydrogenation reactor. After stirring until completely dissolved, add 3.98g Pd(OH) 2 / C (the trade name is palladium hydroxide / carbon, purchased from Beijing Bailingwei Technology Co., Ltd., wherein the palladium content is 20%, the water content is 50%, and the relative molar weight is 0.03). First replace the air in the reactor with nitrogen three times, and then replace the nitrogen in the reactor with hydrogen three times. Under the hydrogen pressure of 0.1MPa, the reactor is heated to 70°C-75°C, and the reaction is continued for 9h.
[0043] Add about 3 cm of diatomaceous earth to the Buchner funnel and moisten it with acetonitrile. After the reaction was completed, the reaction solution wa...
Embodiment 2
[0048] Preparation of N-methyl-N-((3R,4R)-4-methylpiperidin-3-yl)-7H-pyrrolo[2,3-D]pyrimidin-4-amine (compound of formula I): With reference to Example 1, wherein with 95ml concentration is 1mol / L sulfuric acid (with H + Calculate, relative molar weight is 2) replace hydrochloric acid as inorganic acid, replace 175ml acetonitrile with 350ml ethanol as organic solvent, and hydrogen pressure is 0.3MPa. The reaction time was 9 hours, the HPLC purity of the product was 98.98%, and the yield was 83%.
Embodiment 3
[0050] Preparation of N-methyl-N-((3R,4R)-4-methylpiperidin-3-yl)-7H-pyrrolo[2,3-D]pyrimidin-4-amine (compound of formula I): Referring to Example 1, wherein 11.05g of platinum carbon (trade name is platinum carbon, purchased from Beijing Bailingwei Reagent Co., Ltd., the platinum content is 5%, and the relative molar weight is 0.03) replaces palladium carbon as a catalyst. The reaction time is 8 hours, the HPLC purity is 99.32%, and the yield is 92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com