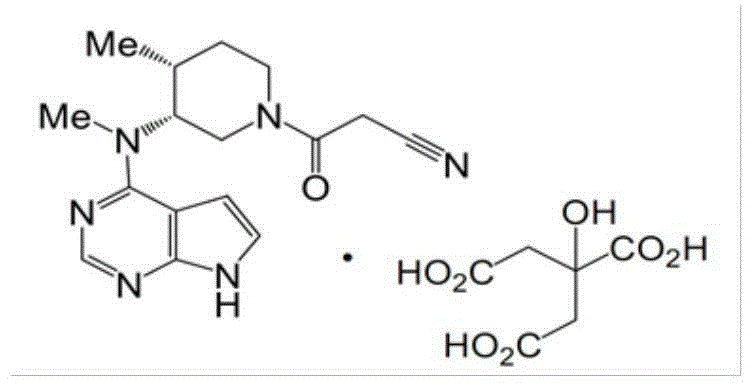
Tofacitinib tablet and preparation method thereof
A technology of tofacitinib and cloth tablets, which is applied in anti-inflammatory agents, pill delivery, pharmaceutical formulations, etc. It can solve the problems of large weight difference, poor powder fluidity, and lobes, and achieve qualified quality, stable properties, and avoid degradation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
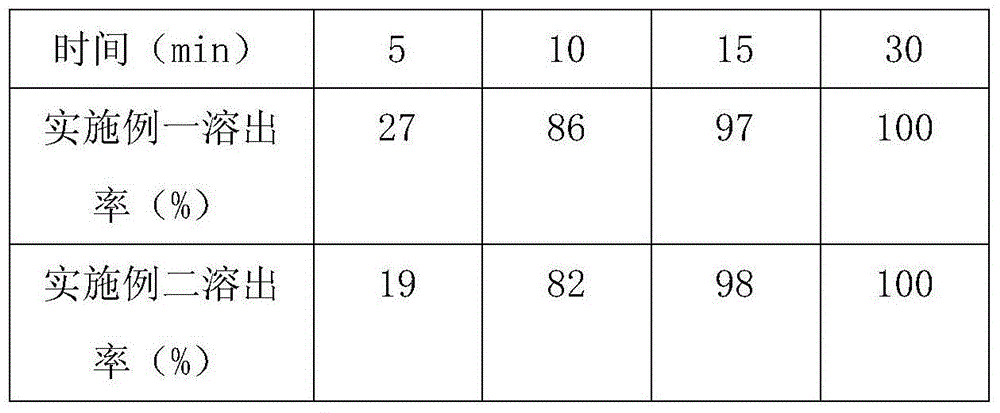
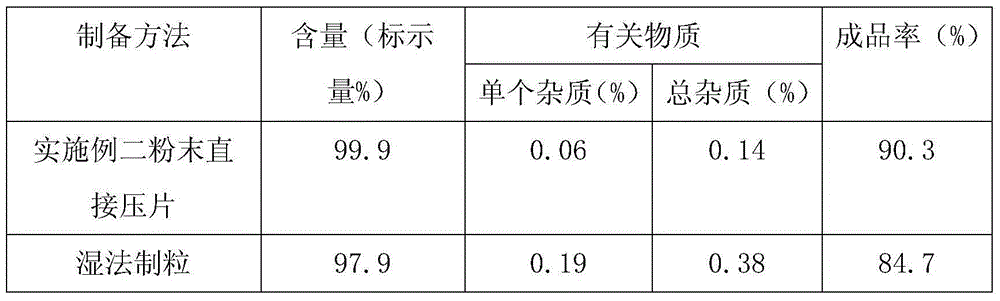
Embodiment 1
[0039] a recipe
[0040] Tofacitinib 5.0g
[0041] Directly compressed lactose 40g
[0042] Microcrystalline Cellulose 50g
[0043] Croscarmellose Sodium 2g
[0045] Coating powder Opadry 33G28523 weight gain 3%
[0046] Specification: 5mg
[0047] Two system method
[0048] (1) Ingredients: Weigh the prescribed amount of tofacitinib, direct-pressed lactose, microcrystalline cellulose, croscarmellose sodium, and magnesium stearate, pass the tofacitinib raw material through a 100-mesh sieve, and set aside .
[0049] (2) Mixing: Mix tofacitinib with direct-pressed lactose, microcrystalline cellulose and croscarmellose sodium for 10-20 minutes to make them uniform, and pass through a 100-mesh sieve.
[0050] (3) Add the prescribed amount of magnesium stearate to the medicated powder mixed uniformly in step (2), and mix for 5 minutes to make it uniform.
[0051] (4) Directly compress the uniformly mixed medicinal powder in step (3), and cont...
Embodiment 2
[0054] The formula is as follows:
[0055] Tofacitinib 10.0g
[0056] Directly compressed lactose 80g
[0057] Microcrystalline Cellulose 25g
[0058] Croscarmellose Sodium 6g
[0059] Magnesium stearate 0.8g
[0060] Coating powder Opadry 33G28523 weight gain 3%
[0061] Specification: 5mg
[0062] For the preparation method, refer to Example 1, and the hardness is controlled within the range of 75-100N.
Embodiment 3
[0064] The formula is as follows:
[0065] Tofacitinib 10.0g
[0066] Directly compressed lactose 120g
[0067] Microcrystalline Cellulose 20g
[0068] Croscarmellose Sodium 6g
[0069] Magnesium stearate 0.8g
[0070] Specification: 5mg
[0071] For the preparation method, refer to Example 1. The fluidity and compressibility of the prepared granules do not meet the requirements of the tableting process.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com