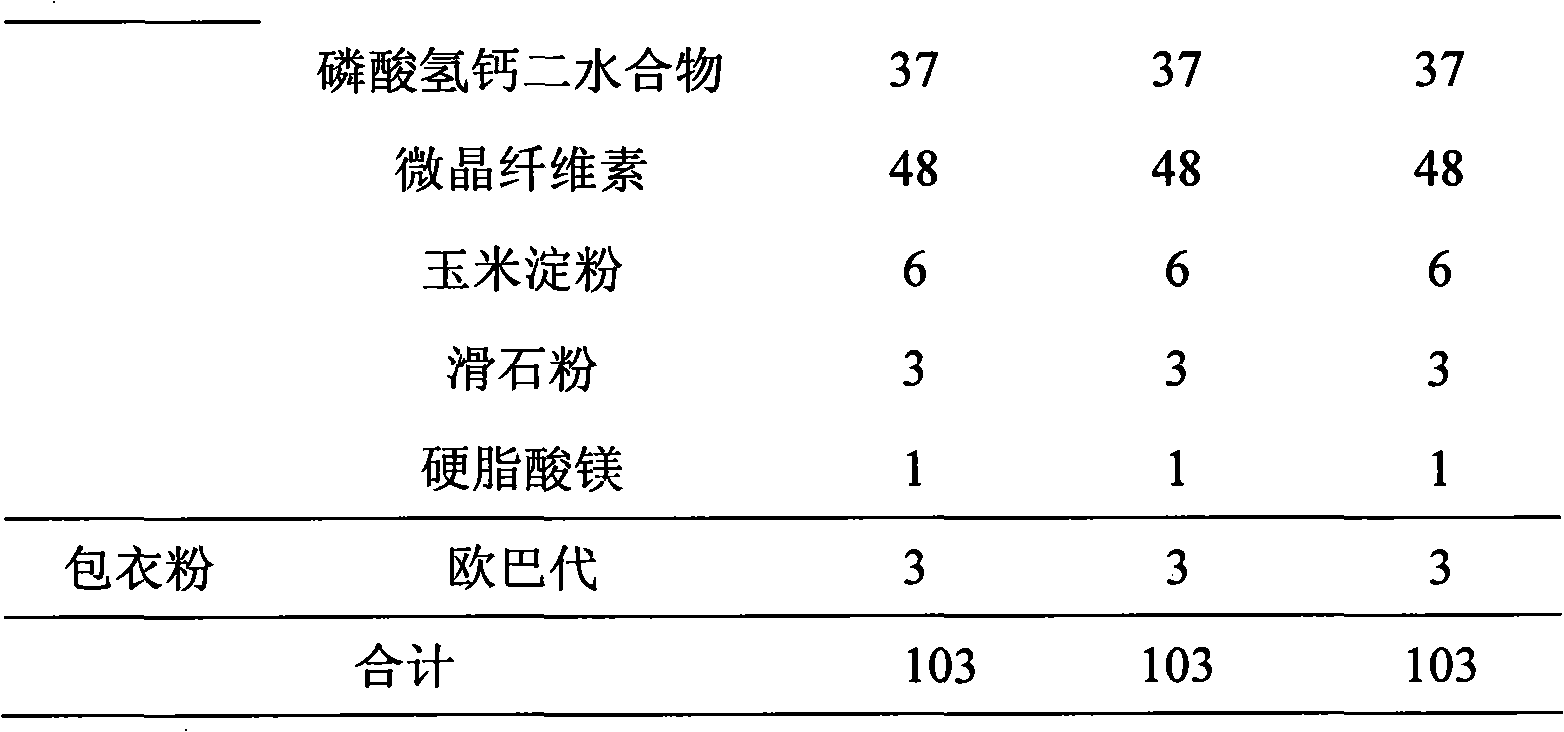
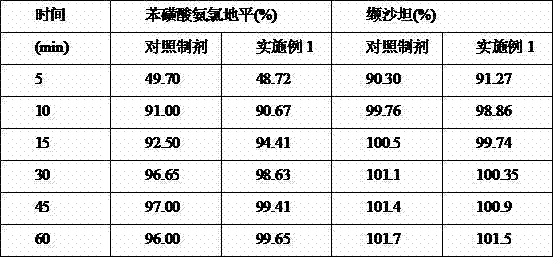
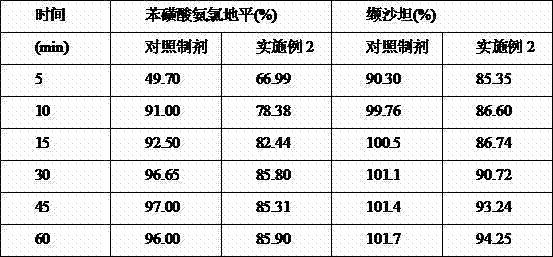
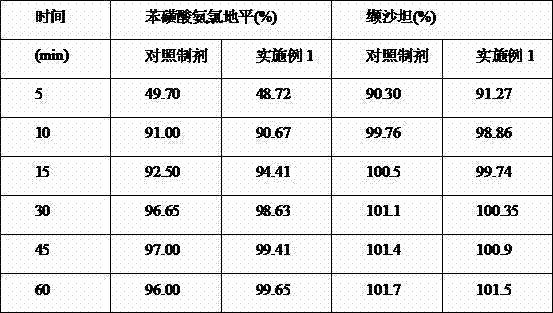
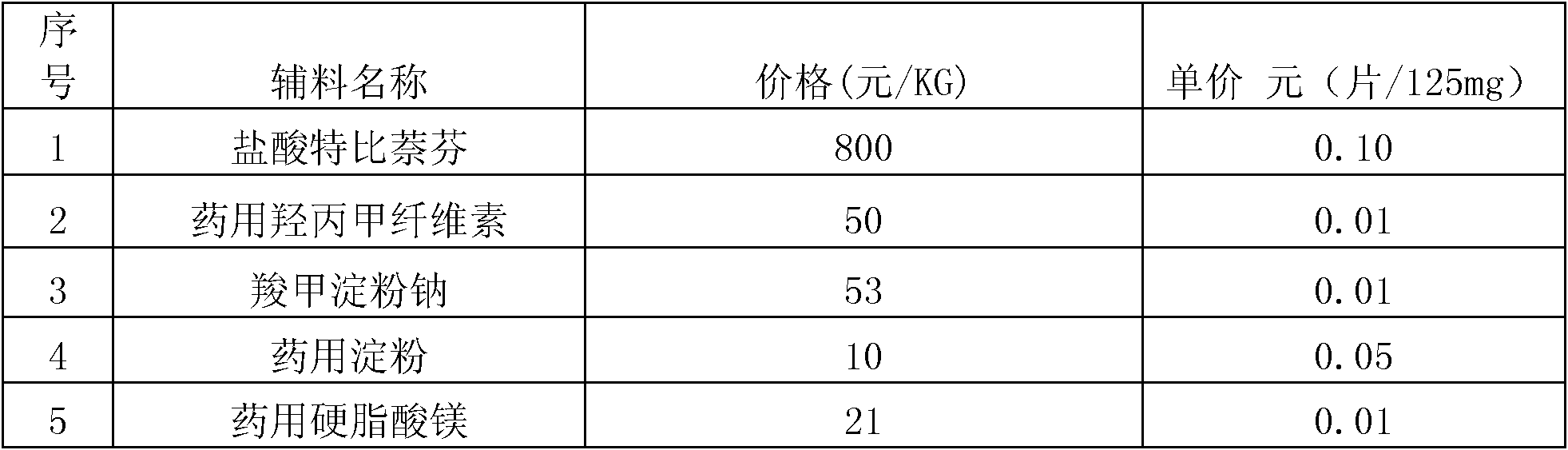
Patents
Literature
48results about How to "Good compression formability" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
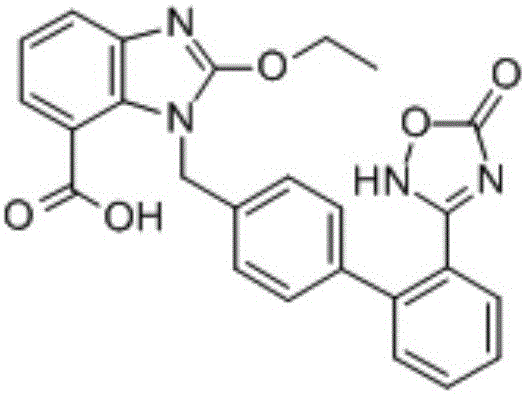
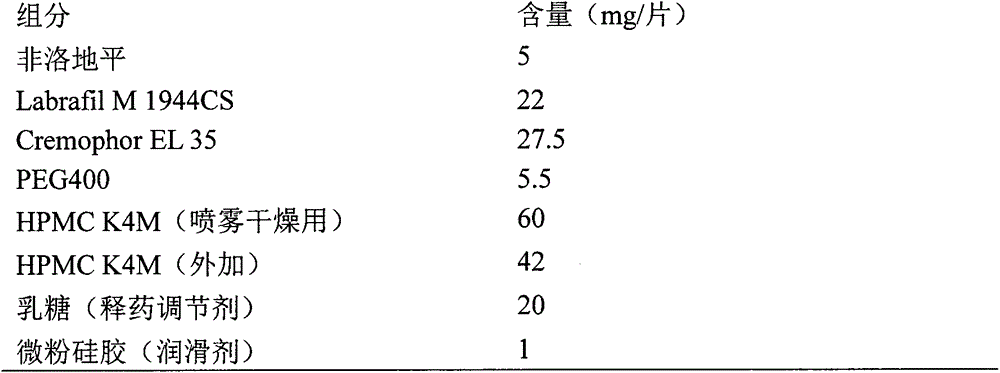
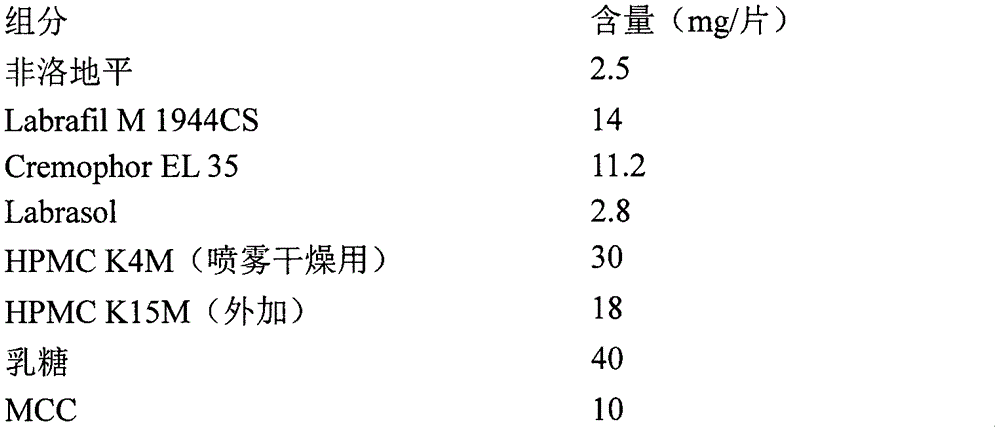
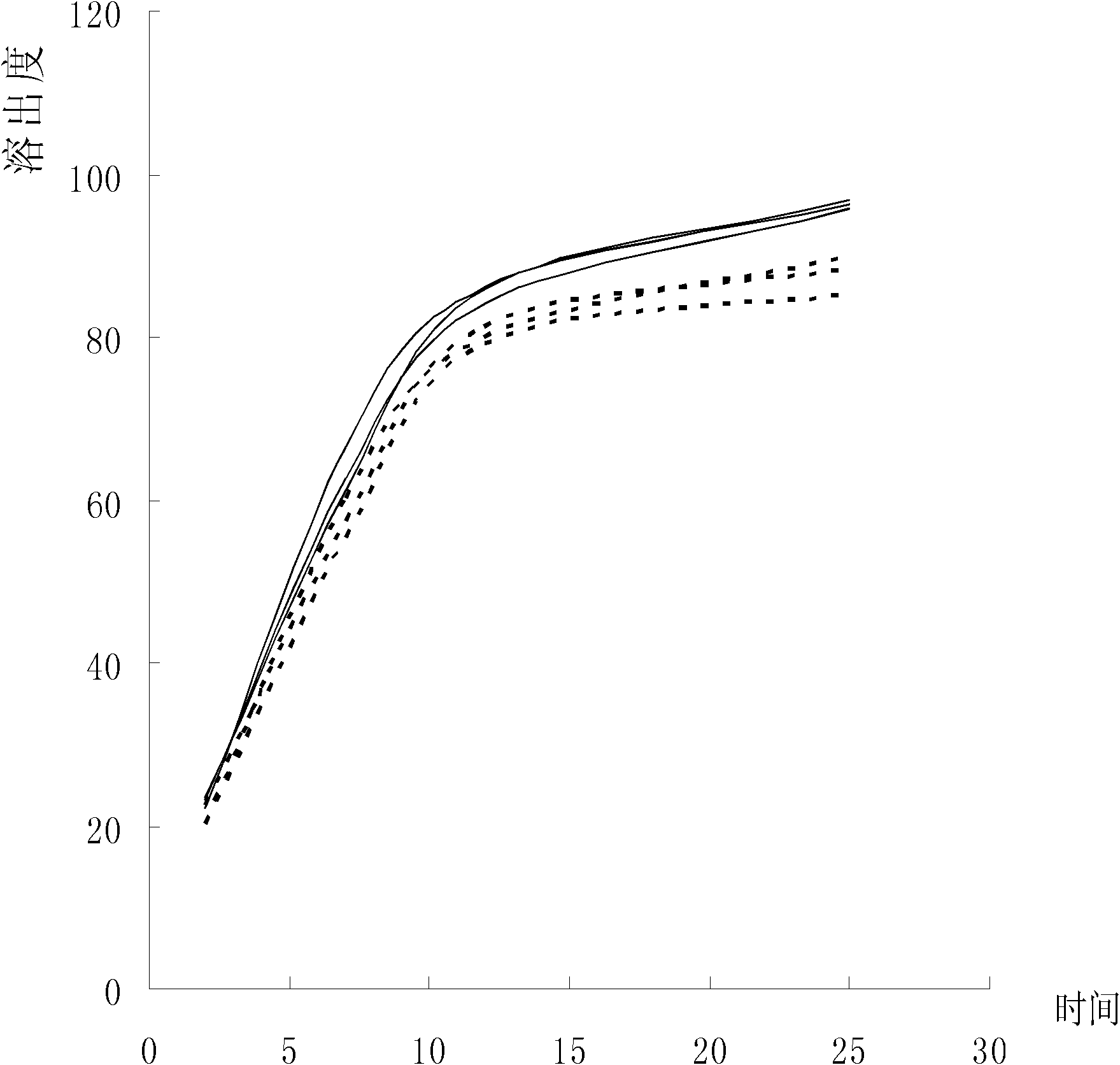
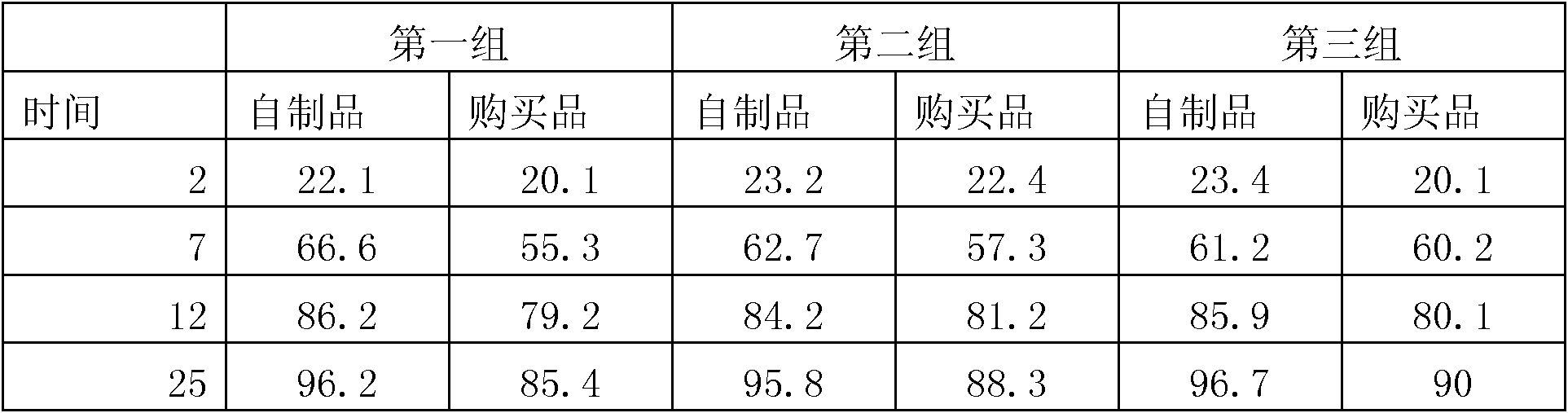
Azilsartan tablets and preparation method thereof
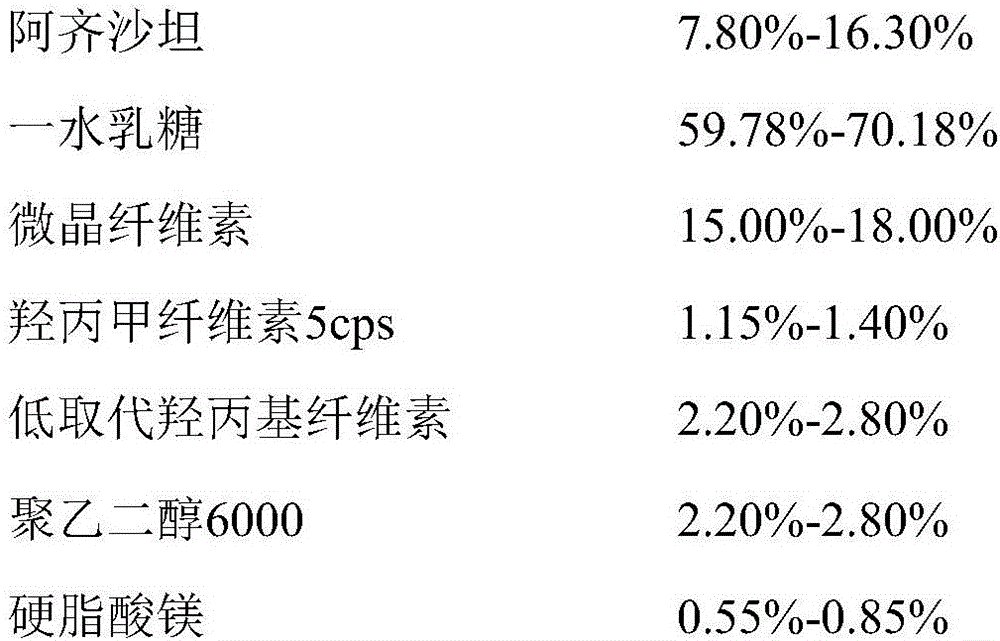
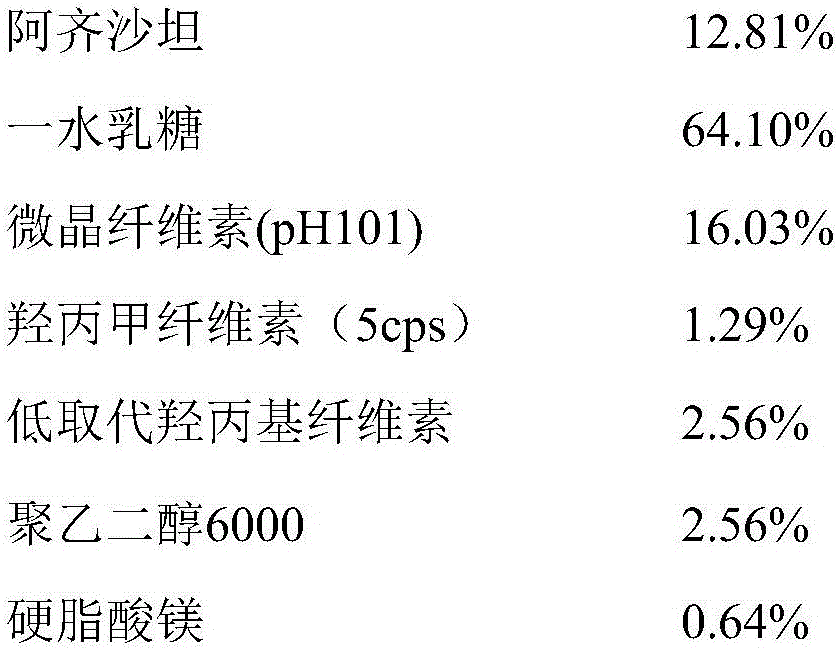
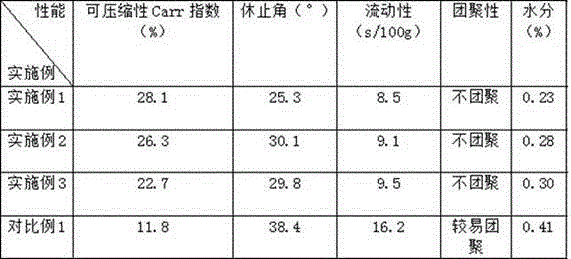
InactiveCN105853384ASolve the hardnessSolve the defect of poor friabilityOrganic active ingredientsPill deliveryLACTOSE MONOHYDRATESpray Granulation
The invention discloses azilsartan tablets and a preparation method thereof, and belongs to the field of pharmacy. For the azilsartan tablets, each azilsartan tablet is prepared from, by mass, 7.80%-16.30% of azilsartan, 59.78%-70.18% of lactose monohydrate, 15.00%-18.00% of microcrystalline cellulose, 1.15%-1.40% of hydroxypropyl methylcellulose (5cps), 2.20%-2.80% of low-substituted hydroxypropyl cellulose, 2.20%-2.80% of polyethylene glycol 6000 and 0.55%-0.85% of magnesium stearate. By means of a fluidized bed top-spraying granulation method, three processes of mixing, granulation and drying in a traditional technology are completed in one step, the production efficiency is improved, and the prepared granules are uniform in granule size and good in fluidity and compression formability; meanwhile, due to the fact that a disintegrating agent is added internally and externally, the problem that the azilsartan tablets disintegrate and dissolve slowly is solved.
Owner:JIANGSU ZHONGBANG PHARMA
Cellooligosaccharide-containing composition
InactiveCN101272794AImprove lipid metabolismEnhanced barrier functionAntibacterial agentsOrganic active ingredientsFiberCellobiose
Owner:ASAHI KASEI KK
Allose tablet excipient, medicinal tablet and method for preparing medicinal tablet
InactiveCN102697744AImprove emulsion stabilityGood thickening effectOrganic active ingredientsInorganic non-active ingredientsSolubilityOctenyl succinate
The invention discloses an allose tablet excipient. The allose tablet excipient consists of the following components in percentage by weight: 88 to 96 percent of allose, 1 to 5 percent of starch octenyl succinate anhydride, 1 to 5 percent of silicon dioxide and 1 to 5 percent of kaolin, is high in fluidity, molding degree and demoulding performance, and can be directly compressed together with medicines and water to obtain medicinal tablets. The invention also discloses a medicinal tablet. The medicinal tablet consists of the following components in percentage by weight: 75 to 85 percent of medicinal active ingredient, 5 to 15 percent of allose tablet excipient and 5 to 15 percent of water raw material. The allose tablet excipient and the medicinal active ingredient do not have incompatibility and are reacted with each other; and the medicinal tablet is high in dissolvability and fluidity and is suitable for directly compressing various medicines. The invention also discloses a method for preparing the medicinal tablet. The method makes the medicinal tablets easily prepared and is easy to implement, operate and control.
Owner:安吉东来药用辅料有限责任公司
Preparation method of solid pharmaceutical composition containing desloratadine
ActiveCN101849902AReduce intensityLow densityOrganic active ingredientsInorganic non-active ingredientsValsartanMedicine
The invention relates to a pharmaceutical preparation containing valsartan, which at least comprises active ingredient valsartan or a pharmaceutically acceptable salt thereof and at least two disintegrants in a ratio between 1: 2 and 2;1. The invention also relates to a preparation method of the valsartan preparation, which comprises the following steps of: firstly, pelletizing the valsartan or the pharmaceutically acceptable salt thereof by a rolling method; mixing with at least two disintegrants and other pharmaceutic accessories; and tabletting or filling capsules. Compared with a product prepared by the prior art, the valsartan preparation of the invention has better stability. The method of the invention has simple production process and high production efficiency and is suitable for industrial production.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
Solid preparation containing amlodipine and valsartan
The invention provides a solid preparation containing amlodipine and valsartan, which is used as a therapeutic drug for hypertension and the like. The amlodipine and the valsartan are within a certain particle diameter ratio range. Thus, the solid preparation containing amlodipine and valsartan has excellent content uniformity, leaching property, hardness and the like.
Owner:BEIJING D VENTUREPHARM TECH DEV
Preparing method for compressible saccharose
ActiveCN105833280AImprove liquidityLow densityInorganic non-active ingredientsGranular deliverySucrose measurementFluidized bed
The invention relates to a preparing method for compressible saccharose .The method comprises the steps that saccharose serves as a main raw material, a crystallizing resisting agent and talcum powder serve as auxiliary materials, a fluidized bed is used for spraying drying, and compressible saccharose particles are obtained .The method is simple in technological process and small in investment and energy consumption; besides, the obtained compressible saccharose is good in compressibility and mobility, high in uniformity and free of agglomeration .
Owner:HUNAN ER KANG PHARMA
Farming and stockbreeding waste fuel forming machine
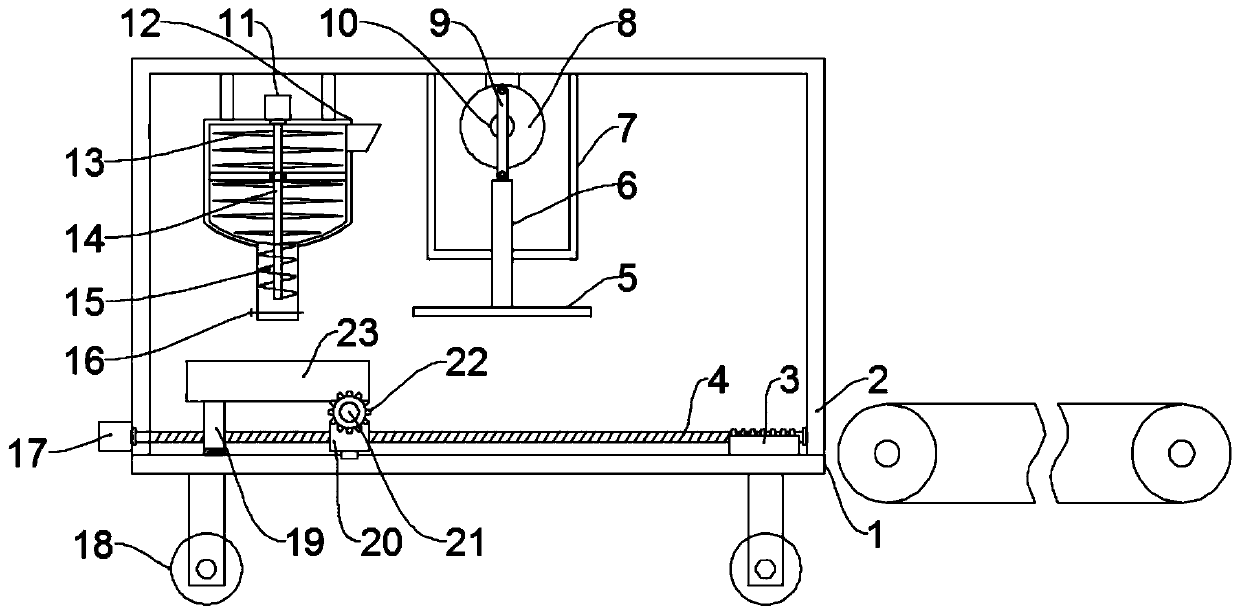
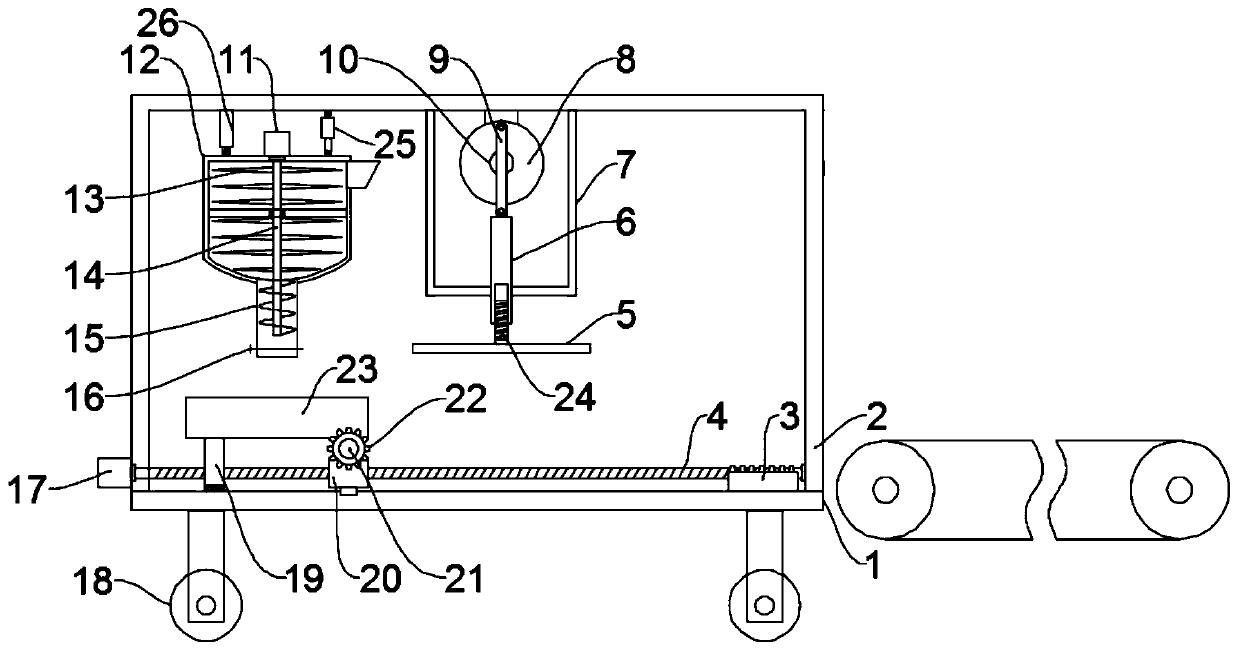
ActiveCN109747208AAvoid manual rotationReduce labor intensityGrain treatmentsShaping pressProduction lineCompression device
The invention discloses a farming and stockbreeding waste fuel forming machine, and relates to the technical field of farming and stockbreeding waste utilization. The problem that existing farming andstockbreeding waste fuel production equipment is simplex in function and requires manual material transfer is mainly solved. The forming machine comprises a substrate and a crushing device and a compression device; a door-type frame is fixed to the upper surface of the substrate; the crushing device is mounted on the door-type frame; the compression device is mounted on one side of the crushing device; a conveying device is arranged at the position, below the crushing device and the compression device, of the upper surface of the substrate; and the conveying device comprises a third motor, afirst threaded rod, a movable block, a collecting frame, a mounting shaft, a gear and a rack. The crushing device, the compression device and the conveying device are arranged, waste can be sequentially conveyed to the position below the crushing device and the compression device through the conveying device, then fed into a conveyor belt to be fed to the next process, production line processing and integrated arranging are achieved, the automation degree is high, the work efficiency is improved, waste manual rotation is avoided, and the labor intensity of staff is relieved.
Owner:LANZHOU UNIVERSITY OF TECHNOLOGY
Indissolvable drug oral sustained-release dry emulsion tablet and preparation method thereof
InactiveCN105168163ADissolution medium volume is smallIncrease speedOrganic active ingredientsPharmaceutical delivery mechanismBlood concentrationDissolution
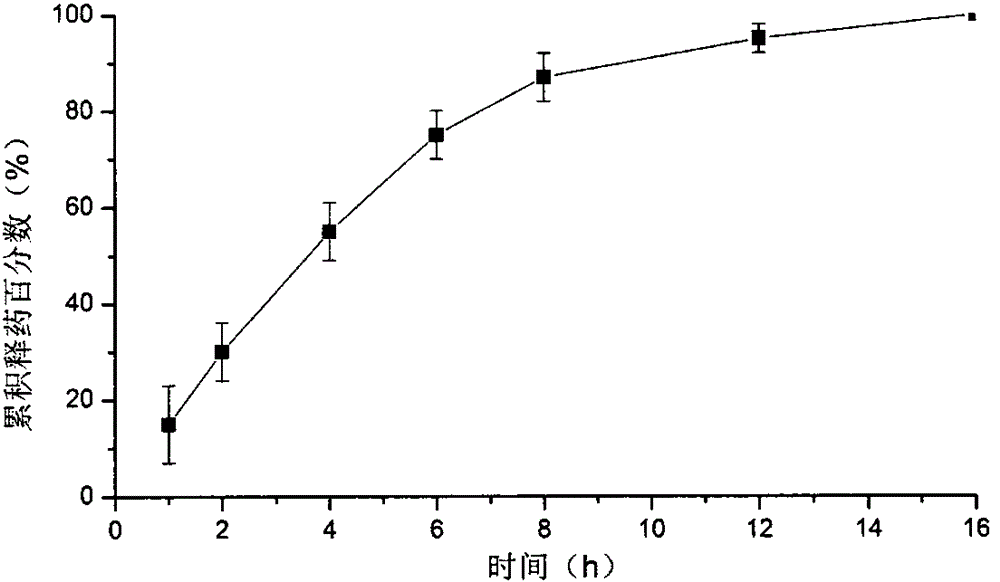
The invention belongs to the field of medicinal preparations, and discloses an indissolvable drug oral sustained-release dry emulsion tablet and a preparation method thereof. The preparation method for the oral sustained-release dry emulsion tablet comprises the steps that after an oil phase, a surface active agent and cosurfactant are evenly mixed, an indissolvable drug is added till the drug is dissolved completely, and accordingly a medicine-carried self-emulsifying system is prepared; after the medicine-carried self-emulsifying system and an aqueous solution of a hydrophilic gel framework material are evenly mixed, spray drying is conducted, and sustained-release dry emulsion powder is obtained; after the sustained-release dry emulsion powder and a conventional tablet auxiliary material needed for preparation are mixed, wet granulation is carried out; at last, tabletting is performed. The sustained-release dry emulsion tablet can improve the dissolution and dissolving-out performance of the indissolvable drug, the bioavailability of the indissolvable drug is improved, a stable blood concentration is formed, and the compliance of patients is improved. The sustained-release dry emulsion tablet is not complex in composition of prescription, and the preparation technology is suitable for industrial production.
Owner:CHINA PHARM UNIV
Preparation method of antifungal tablet
InactiveCN102198111ADisintegrates quicklyDisintegration decreasedOrganic active ingredientsAntimycoticsSolubilityAntifungal
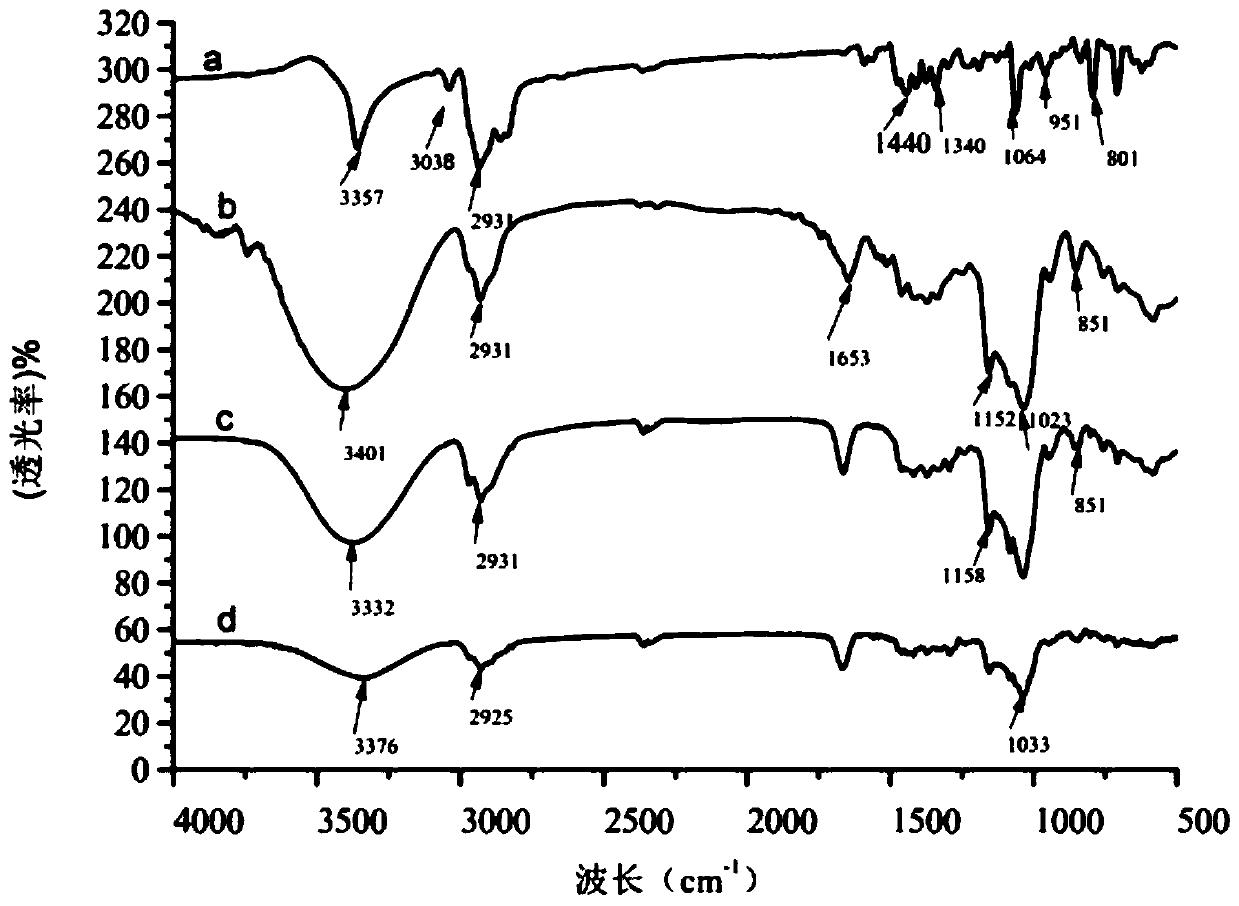
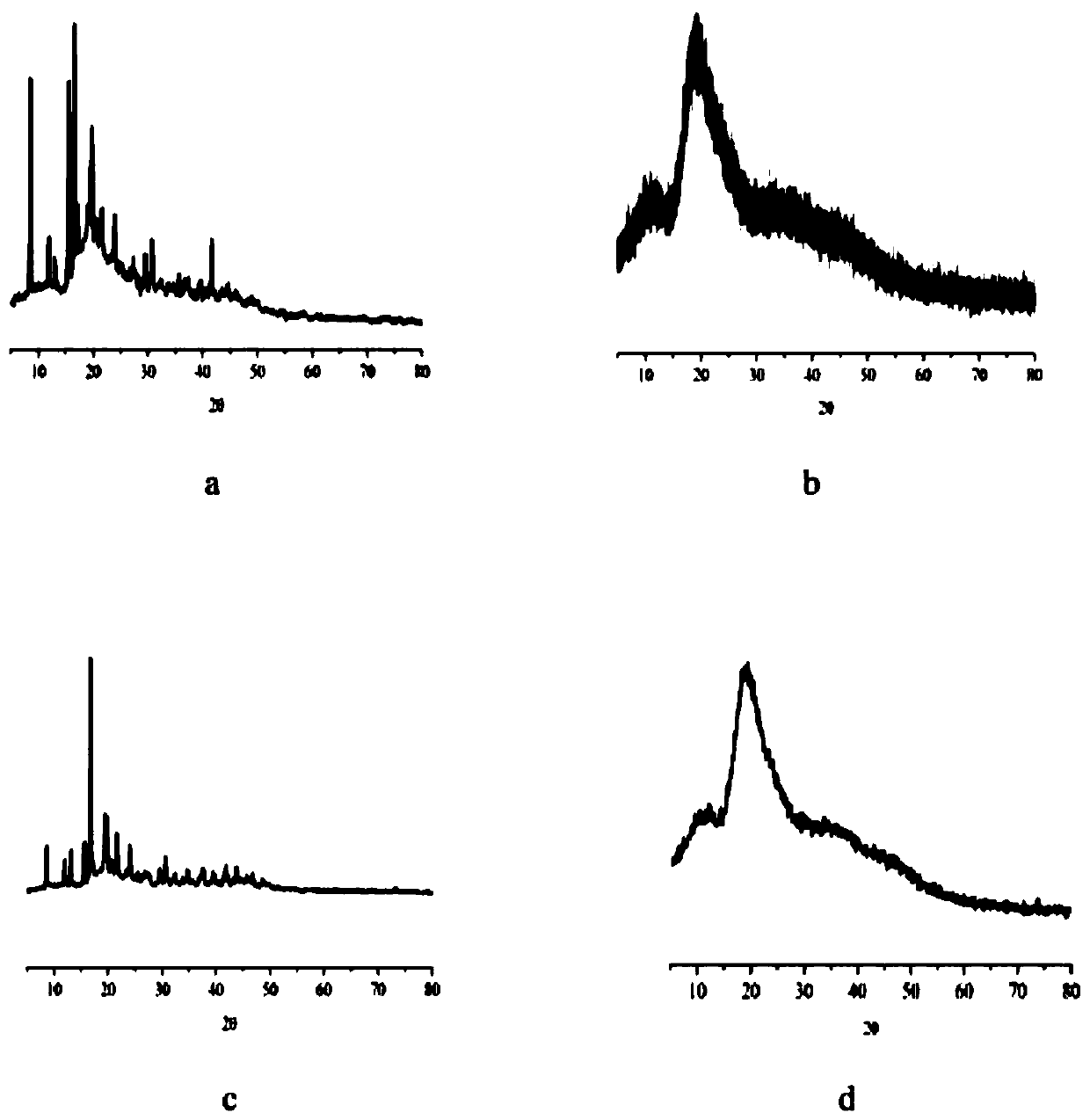
The invention discloses a preparation method of an antifungal tablet and solves the problems of complex process, high cost and high quality control difficulty in the preparation of traditional Terbinafine hydrochloride tablets. The method comprises the steps of: 1, weighing Terbinafine hydrochloride, adhesive, carboxyrnethyl starch sodium, starch and magnesium stearate; and 2, putting and mixing the Terbinafine hydrochloride, the starch and a part of carboxyrnethyl starch sodium in a mixing tank, then adding and mixing the adhesive with the mixture, pelletizing, drying and finishing, adding the magnesium stearate and the rest carboxyrnethyl starch sodium, mixing and tabletting. In the preparation method of the antifungal tablet provided by the invention, by the use of the composite adhesive, the compressibility of drug particles and promote drug disintegration can be enhanced, tight tabletting and good adhesive force are obtained, the obtained tablets are bright and free from sticking, the degree of disintegration is remarkably reduced, the drug solubility is improved, the preparation process is simple in implementation, the cost of raw materials and accessories for every tablet is less than 1 Yuan, therefore, the price is low, and the product quality can be easily controlled.
Owner:哈尔滨乐泰生物科技有限公司
Abiraterone inclusion compound tablet and preparation method thereof
ActiveCN110141556AAccurate doseQuality improvementOrganic active ingredientsPharmaceutical non-active ingredientsSolubilityAdhesive
The invention discloses an abiraterone inclusion compound tablet and a preparation method thereof, and belongs to the technical field of pharmaceutical preparations. The tablet contains an abirateroneinclusion compound and medicinal auxiliary materials. The preparation method comprises the following steps: sieving the abiraterone inclusion compound, a diluent and a disintegrant with a 80-mesh sieve, and performing uniform mixing by an equal-amount increment method; adding an adhesive to prepare a soft material, sieving the obtained soft material with a 14-mesh sieve, preparing wet particles,putting the wet particles into a 60 DEG C oven, performing drying until the moisture content is 1-2%, performing sieving with a 16-mesh sieve, and weighing the dry particles; and adding a lubricant which is 1% by weight of the dry particles, and performing uniform mixing and tabletting. The method prepares the abiraterone inclusion compound into a tablet. The tablet has the advantages of accuratedosage, stable quality, low cost, convenient carrying and the like, is a preferred dosage form of the pharmaceutical preparation, and solves and improves the technical problems of compressibility andsolubility of the abiraterone tablet.
Owner:李建恒
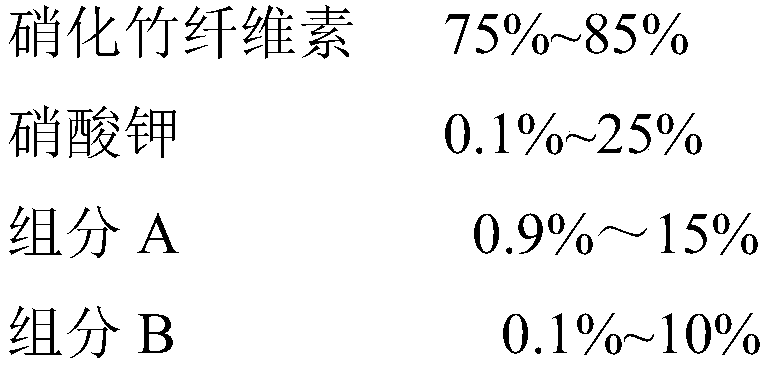
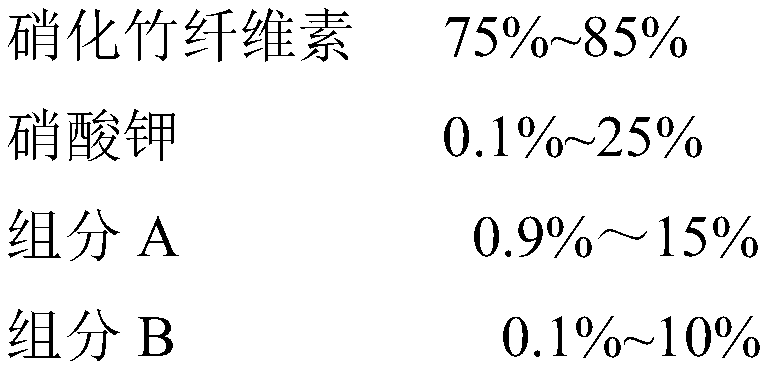
Lift-off agent for safe aerial shells and preparation method thereof
InactiveCN111423288ASimple preparation processEasy to operateExplosive working-up apparatusNon-explosive/non-thermic compositionsCellulosePotassium nitrate
The invention relates to a lift-off agent for safe aerial shells and a preparation method thereof, belonging to the field of pyrotechnic compositions. The agent comprises the following components: nitrified bamboo cellulose, potassium nitrate, a component A and a component B, wherein the component A is any one selected from melamine and dicyandiamide; and the component B is any one selected from guanidine salt, aminoguanidine salt, diaminoguanidine salt and triaminoguanidine salt. The preparation method is simple in process and easy to operate, a high-temperature pressurizing and depressurizing device is not used in the preparation process, operation is safe and reliable, and industrial production can be achieved. The yield of the prepared agent is high, and particles of the produced lift-off agent for safe aerial shells are uniform in particle size and good in compression formability.
Owner:江西吉润花炮新材料科技有限公司
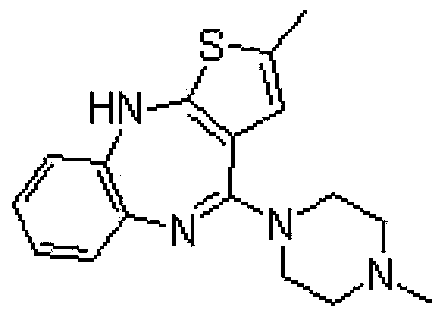
Olanzapine tablet composition and preparation method thereof
ActiveCN104208031AIncrease disintegration speedImprove dissolution rateOrganic active ingredientsNervous disorderAdhesiveDiluent
The invention provides a novel olanzapine tablet composition and a preparation method thereof. Lactose and microcrystalline cellulose, which have the advantages of good fluidity and compressive forming ability, are respectively taken as the diluent and dry adhesive. A simple direct powder press method is taken to prepare the olanzapine tablet, the technology is simple, thus time and energy are both saved, moreover the disintegrating or dissolving speed of the tablet is increased, the tablet bio-utilization degree is also improved, the quality of the prepared olanzapine tablet composition is controllable, and the stability of the olanzapine tablet composition is guaranteed.
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD
Ursodesoxycholic acid capsule and preparation method thereof
PendingCN111135153ARich choiceImprove uniformityOrganic active ingredientsDigestive systemCholic acidEthylic acid
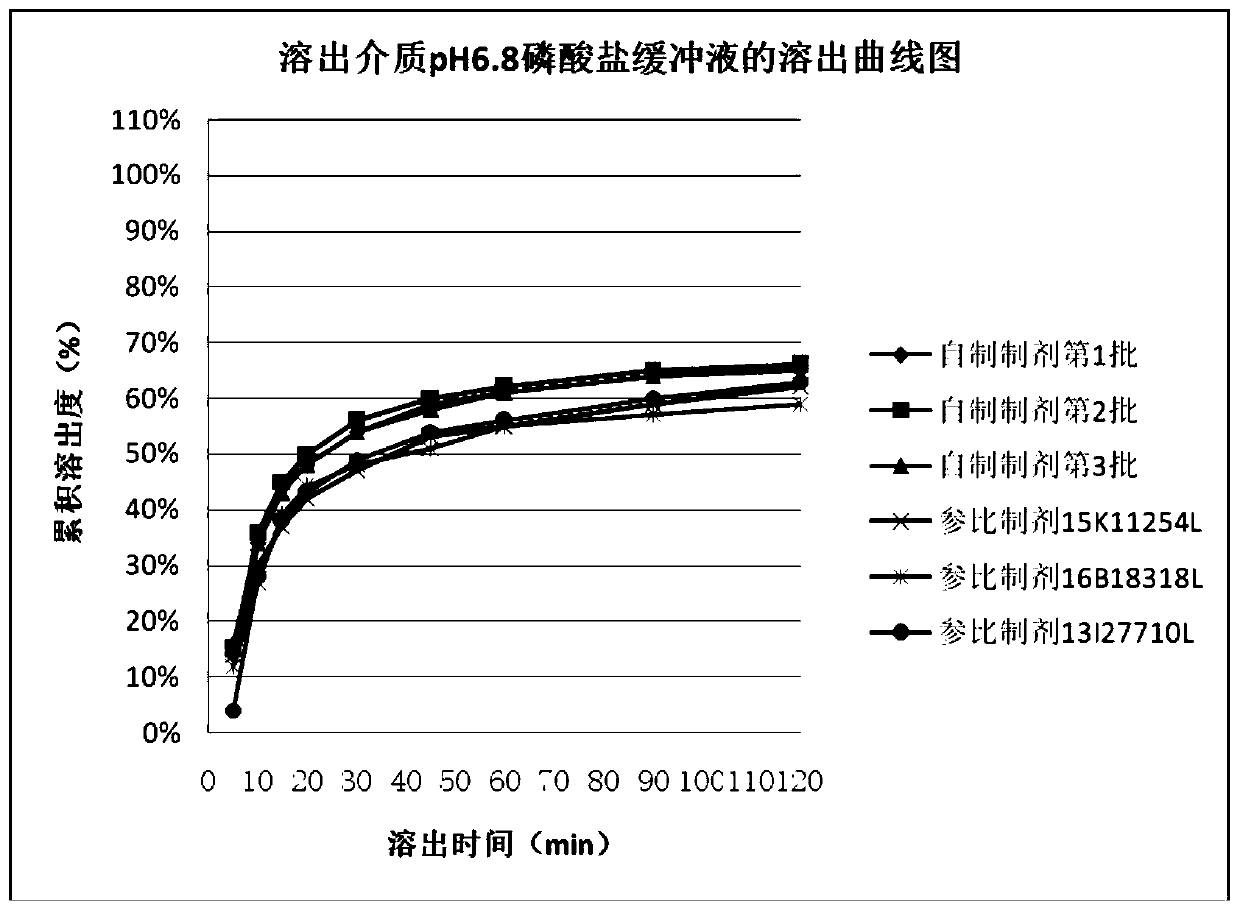
The invention discloses an ursodesoxycholic acid capsule and a preparation method thereof. The ursodesoxycholic acid capsule comprises a content and a capsule shell, wherein the content comprises ursodesoxycholic acid, filling agents, an adhesive and lubricants; the filling agents are microcrystalline cellulose and lactose; the adhesive is povidone K30; and the lubricants are magnesium stearate and silicon dioxide. The ursodesoxycholic acid capsule is obtained by mechanically crushing an ursodesoxycholic acid raw material, the raw material does not need to be micronized, the process is simpleand controllable, types and amounts of the filling agents and the lubricants are optimally selected, parameters of a one-step pelletizing process are optimized, and the prepared ursodesoxycholic acidcapsule product is good in dissolution, small in inter-batch difference and stable in quality, and has dissolution behaviors identical to those of a reference parameter, namely Ursofalk, in five different mediums of a hydrochloric acid solution of which the pHvalue is 1.2, an acetate buffer of which the pH value is 4.5, a phosphate buffer of which the pH value is 6.8, a phosphate buffer of which the pH value is 7.2 and a phosphate buffer of which the pH value is 7.5.
Owner:安士制药(中山)有限公司
Rhinitis granules and preparation method thereof
InactiveCN108743738ANo side effectsReduce body angerAntibacterial agentsDispersion deliveryFlos chrysanthemiAllergy
The invention discloses rhinitis granules and a preparation method thereof, and belongs to the field of traditional Chinese medicine preparation. The rhinitis granules are characterized by being prepared from the following Chinese herbal medicines in parts by weight: 8-20 parts of flos lonicerae, 8-20 parts of flos chrysanthemi indici, 8-20 parts of taraxacum, 8-20 parts of herba violae, 10-25 parts of fructus xanthii, 5-18 parts of radix semiaquilegiae, 5-18 parts of radix angelicae, 5-18 parts of flos magnoliae, 5-18 parts of herba menthae, 5-18 parts of rhizoma Chuanxiong, 5-18 parts of radix paeoniae rubra, 5-18 parts of radix scutellariae, 5-18 parts of radix glycyrrhizae and 5-18 parts of herba patriniae. The rhinitis granules are convenient to carry and convenient and safe to drink,taste proper, also have effects of resisting bacteria, diminishing inflammation, controlling allergy and improving the immune function of the body, and most importantly, the rhinitis granules are suitable for people with diabetes, people with three highs (high blood pressure, high blood glucose and high blood lipid), pregnant women, children and the like.
Owner:桂平市蒙圩镇火炎种养专业合作社
Isomaltose hypgather tablet excipient, medicine tablet and preparation method
InactiveCN102671200AImprove emulsion stabilityGood thickening effectPill deliveryPharmaceutical non-active ingredientsIsomaltooligosaccharideMedicine
The invention discloses isomaltose hypgather tablet excipient, which consists of 88 percent to 96 percent of isomaltose hypgather, 1 percent to 5 percent of starch octenyl succinate anhydride, 1 percent to 5 percent of silicon dioxide and 1 percent to 5 percent of porcellanite in percentage by weight, and has the advantages that the mobility is good, the forming degree is good, the demolding performance is good, and isomaltose hypgather tablet excipient can be directly tableted with medicine and water for preparing medicine tablets. The invention also discloses a medicine tablet, which consists of 75 percent to 85 percent of medicine active ingredients, 5 percent to 15 percent of isomaltose hypgather tablet excipient and 5 percent to 15 percent of water raw materials in percentage by weight, wherein the isomaltose hypgather tablet excipient and the medicine active ingredients do not have incompatibility and reaction, in addition, the solvability is good, the flowability is good, and the medicine tablet is suitable for the direct tableting of various kinds of medicine. The invention also discloses a preparation method of the medicine tablet, which has the advantages that the preparation is simple, the implementation is easy, and the operation and the control are easy.
Owner:安吉东来药用辅料有限责任公司
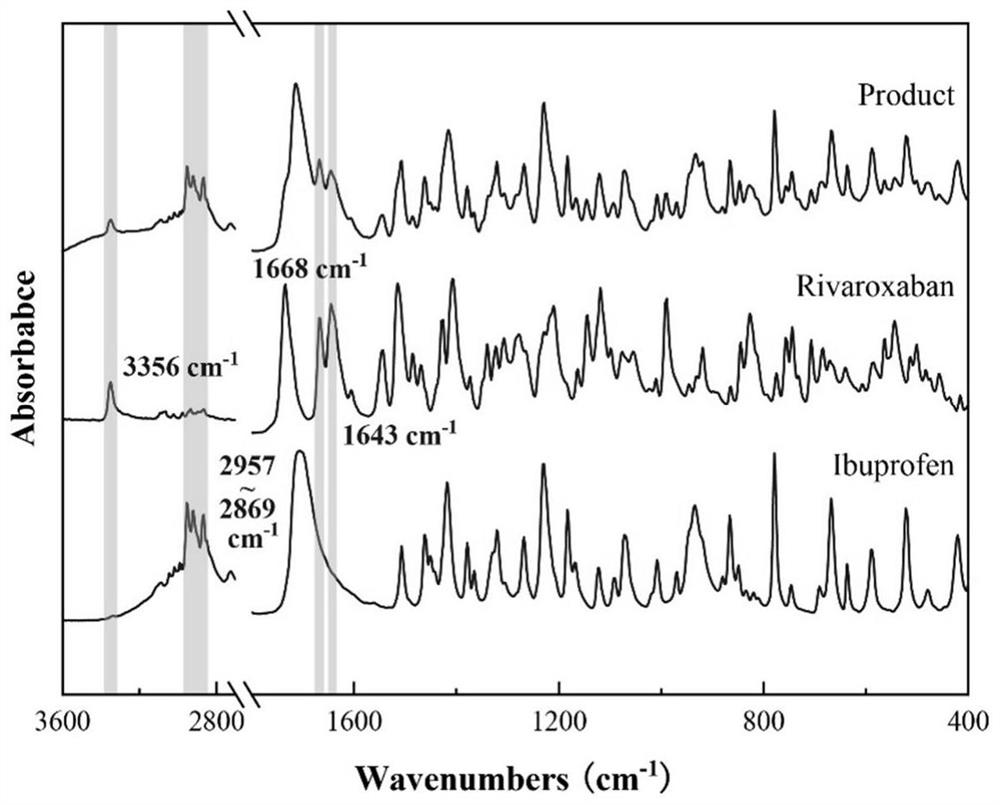
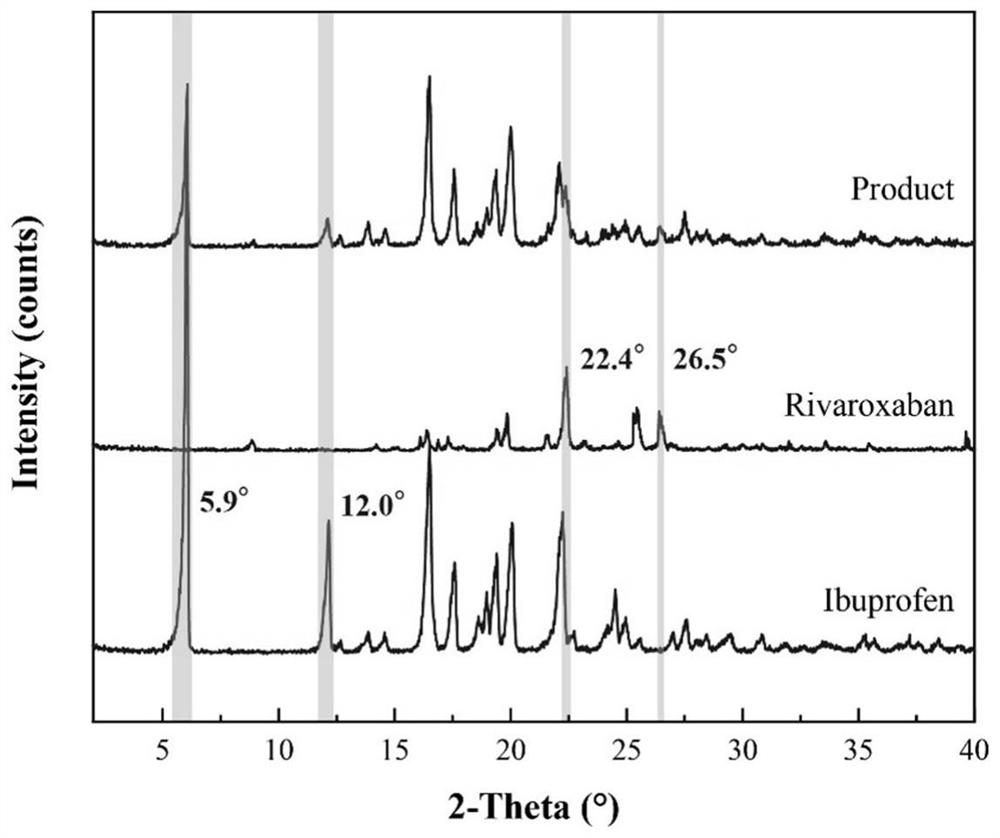
Ibuprofen-loaded rivaroxaban functional particles and preparation method thereof
ActiveCN112915089AMeet the requirementsComponent content is controllableOrganic active ingredientsAntipyreticPostoperative complicationRivaroxaban
The invention provides ibuprofen-loaded rivaroxaban functional particles and a preparation method thereof. The functional particles are spherical composite particles, and the mass ratio of ibuprofen to rivaroxaban in the functional particles is (0.76-3.80): 1. The ibuprofen-loaded rivaroxaban functional particles have good uniformity and fluidity, are not easy to coalesce, have an adjustable ratio of components, and have a good application prospect in the aspect of postoperative complication prevention of hip or knee replacement surgeries, and ibuprofen and rivaroxaban both exist in a stable crystal form. The ibuprofen-loaded rivaroxaban functional particles are simple in preparation method, low in price, easy to implement, free of auxiliary materials, round and full in particle, low in energy consumption, high in efficiency, green, environmentally friendly, highly mixed, capable of achieving industrialization and low in economic investment, and only water is used as a solvent.
Owner:TIANJIN UNIV
Method of producing copper-tin 33 alloy powder for glass grinding tool
The invention discloses a method of producing a copper-tin 33 alloy powder for a glass grinding tool. The iron-cobalt-copper alloy powder is dendritic; in terms of weight percentage, the cobalt content accounts for 95-99%, and the chromium content accounts for 1-5%; the iron-cobalt-copper alloy powder has the fisher particle size of 1.0 to 1.2 micrometers; the oxygen content is less than 0.50%; and the apparent density is 0.4 to 0.6g / cm<3>. The yield of the method is more than 95%; the yield of the traditional atomization method is only 60-70%; the method can greatly reduce the energy consumption; the cobalt carbonate and the chromium carbide used as raw materials in the production process of the method are melted and cracked at a high temperature and the chemical components are decomposed, meanwhile crystals formed by mutual penetration have wide distribution of elements, uniform voidage and high degree of forming, and effectively improves the rupture strength and service life of theproduct, and the co-melting of the crystals with other metals is improved.
Owner:江苏萌达新材料科技有限公司
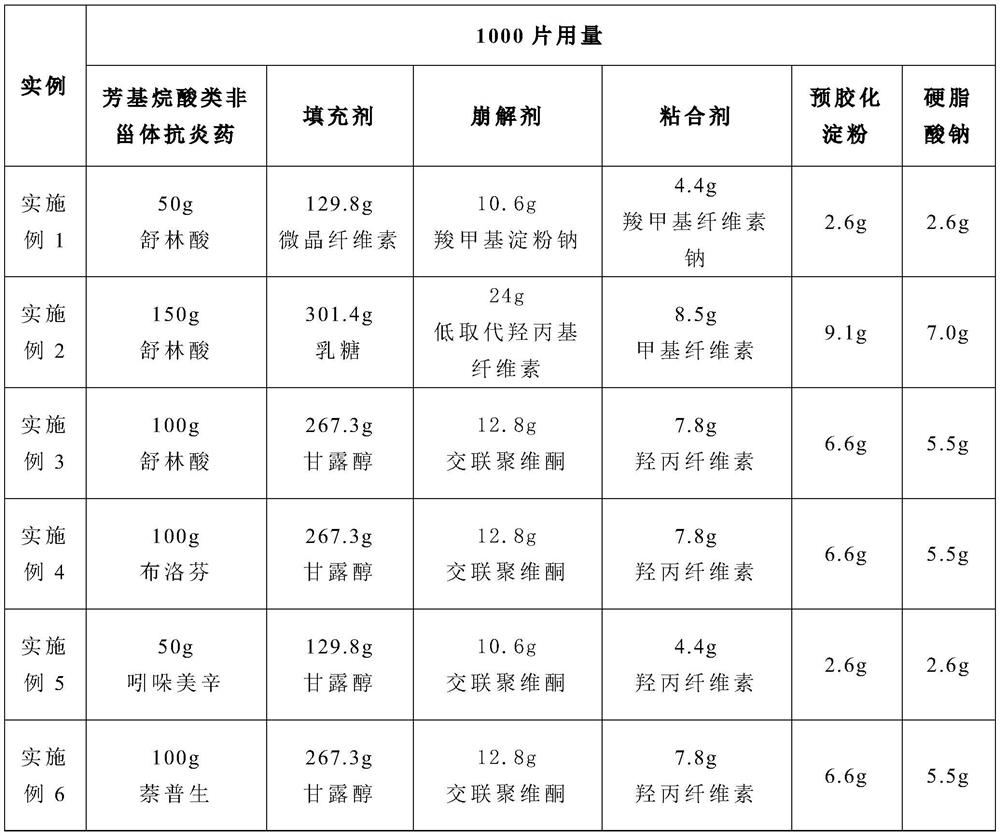
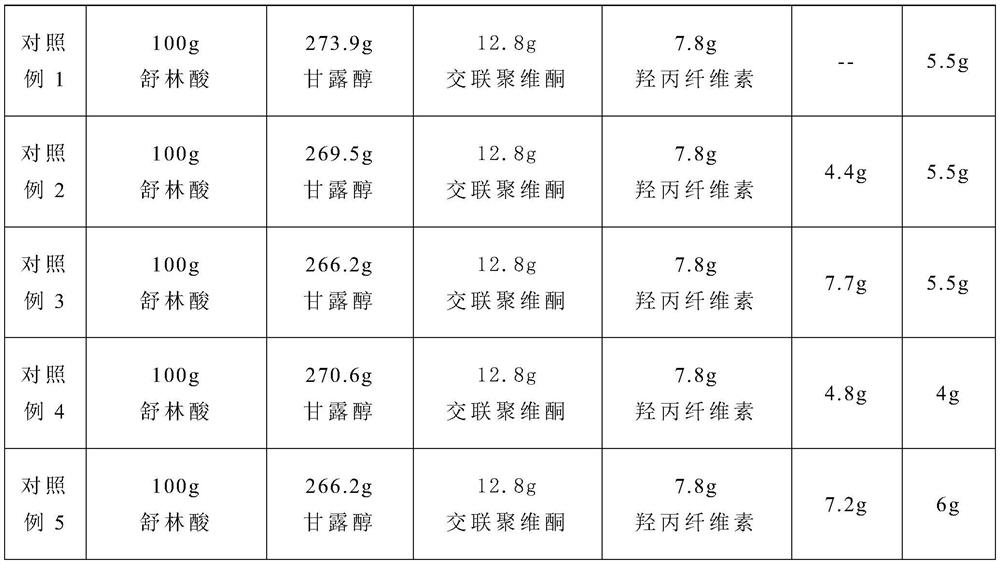
Medicament composition of a high-load lubricant and preparation method thereof
ActiveCN111150710BHigh tensile strengthGood compression formabilityOrganic active ingredientsAntipyreticSodium stearateMedicine
The invention provides a high-load lubricant pharmaceutical composition and a preparation method thereof. The high-load lubricant pharmaceutical composition includes the following ingredients in parts by weight: at least one aryl alkanoic acid non-steroidal anti-inflammatory drug 50 -150, at least one filler 120-350, at least one disintegrant 10-30, at least one binder 4-10, wherein the pharmaceutical composition also includes a lubricant, the lubricant is in the pharmaceutical combination 2.3-4% of the mass percentage in the product; the lubricant is a mixture of pregelatinized starch and sodium stearate with a weight ratio of 1-1.3:1; Sodium fatty acid and pregelatinized starch are used as lubricants, and their dosage is screened, which not only makes the dissolution and disintegration of the pharmaceutical composition meet the regulations, but also significantly improves the tensile strength of the pharmaceutical composition, thereby improving its compression formability .
Owner:于广华
Pyrazinamide tablet and preparation method thereof
InactiveCN109106692AUniform particle sizeGood fluidity and compression moldabilityAntibacterial agentsOrganic active ingredientsPelletizingCorn starch
The invention belongs to the field of pharmaceutic preparations and specifically relates to a pyrazinamide tablet and a preparation method thereof. The ingredient of the pyrazinamide tablet provided by the invention comprises corn starch, pre-gelled starch and magnesium stearate. The preparation method comprises the following steps: adopting a fluidized bed one-step pelletizing process for preparing grains, and then additionally adding lubricant magnesium stearate, thereby obtaining the pyrazinamide tablet. According to the method provided by the invention, the grains with uniform size, excellent liquidity and compression molding property can be prepared on the basis of the reducing of ingredient dosage, and the pyrazinamide tablet with low weight and stable product quality is finally prepared. The pyrazinamide tablet prepared according to the method provided by the invention is beneficial to the intake of patients and is capable of obviously enhancing the adaptability of the patientsso as to reduce the drug resistance. The fluidized bed pelletizing process is capable of saving production time and meets the requirements of GMP specification. The quality of the prepared pyrazinamide tablet is controllable and the stability of the products is guaranteed.
Owner:CHONGQING HUAPONT PHARMA
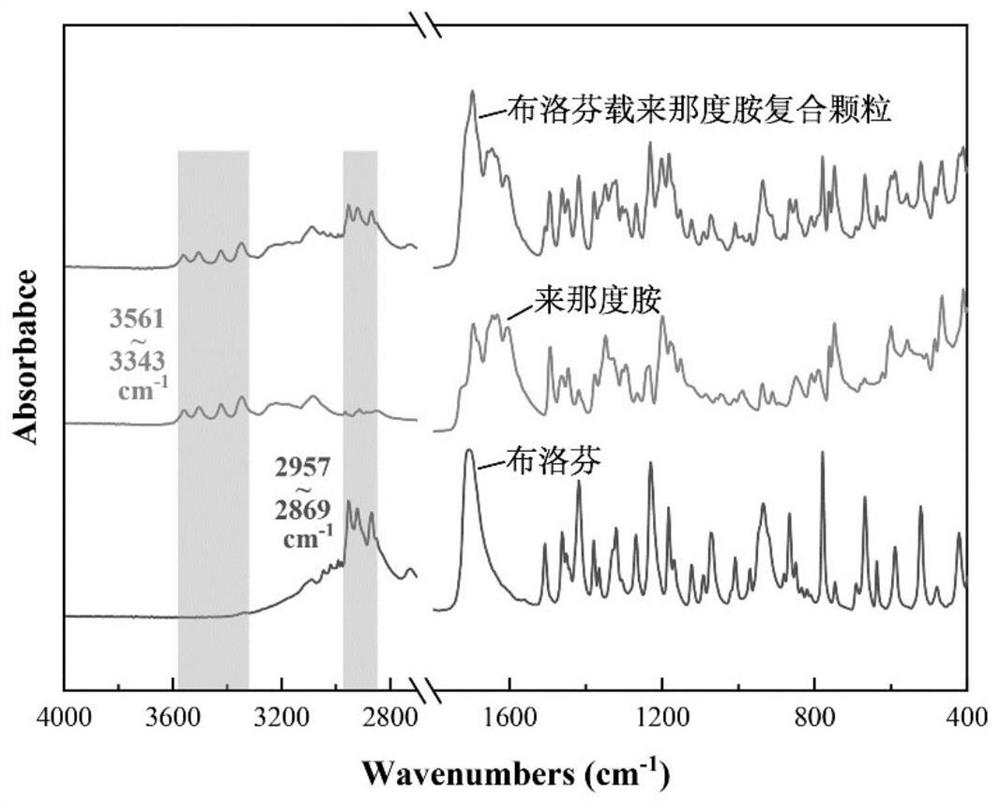
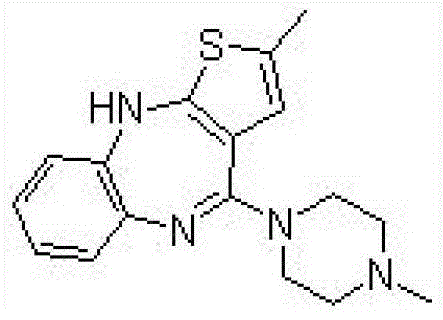
Ibuprofen-loaded lenalidomide composite particle and preparation method thereof
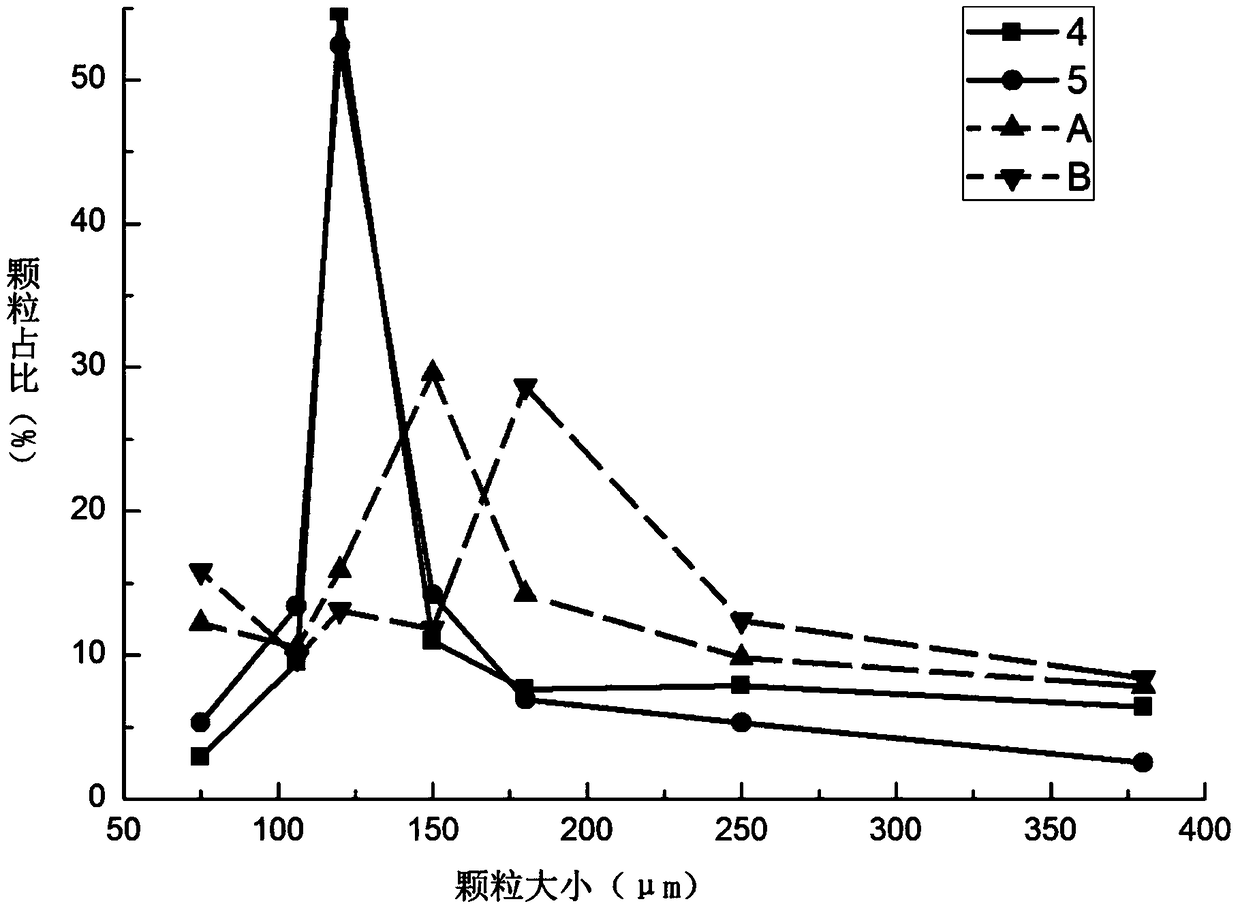
ActiveCN112999224AMeet the requirementsComponent content is controllableOrganic active ingredientsAntipyreticPhysical chemistryNanotechnology
The invention provides an ibuprofen-loaded lenalidomide composite particle and a preparation method thereof, wherein the composite particle is a spherical composite particle, and the mass ratio of ibuprofen to lenalidomide in the composite particle is (0.55-3.5):1. The ibuprofen-loaded lenalidomide composite particle has good uniformity and fluidity and is not easy to coalesce, an proportion among the components is adjustable, ibuprofen and lenalidomide both exist in stable crystal form, and the composite particle has good application prospects in treatment of tumors and complications thereof such as fever. The preparation method of the ibuprofen-loaded lenalidomide composite particle is simple, low in price, easy to implement, free of auxiliary materials, round and full in particle, low in energy consumption, high in efficiency, green, environmentally friendly, highly mixed, capable of achieving industrialization and low in economic investment, and only water is used as a solvent.
Owner:TIANJIN UNIV
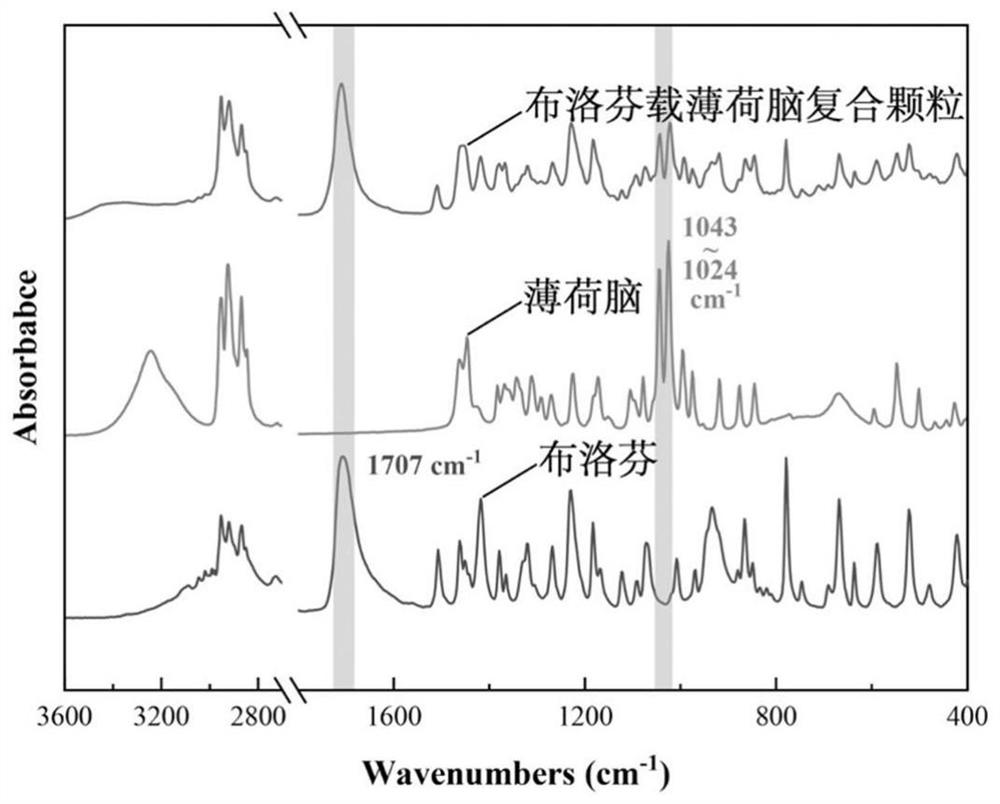
Ibuprofen-loaded menthol composite particle and preparation method thereof
ActiveCN112999170AMeet the requirementsWell mixedHydroxy compound active ingredientsAntipyreticMentholTherapeutic effect
The invention provides an ibuprofen-loaded menthol composite particle and a preparation method thereof, wherein the composite particle is a spherical composite particle, and the mass ratio of ibuprofen to menthol in the composite particle is (0.05-5.67):1; the ibuprofen-loaded menthol composite particle has good uniformity, flowability and high stability, and can be directly tableted, so that the granulation cost is saved; the ibuprofen-loaded menthol composite particle has a good treatment effect on patients with fever and inflammation, the preparation method is simple, the energy consumption is low, the efficiency is high, only water is used as a solvent, the raw material is single, the ibuprofen-loaded menthol composite particle is green and environment-friendly, industrialization can be realized, and the economic investment is low.
Owner:TIANJIN UNIV
Composite bread premixed flour
PendingCN110402984AImprove network structureImprove qualityDough treatmentDough/pre-mixesCelluloseMannitol
The invention discloses composite bread premixed flour, and belongs to the technical field of food processing. According to the composite bread premixed flour, konjac powder is used as a raw material,through the carboxymethylation action, then the konjac powder is blended with multiple components, a network-like structure formed by the konjac powder as the main component is improved, mannitol andmicrocrystalline cellulose can be cooperated to regulate the hydrophily, the viscoelasticity is stabilized, and the elasticity and taste of bread prepared by the premixed flour are improved by combination of biological bacteriostasis and high thermal stability components; and peanuts are dipped and germinated for double cultivation, active components and oil are collected after filter pressing, in the process of subsequent mixing with other components, activated organic matter can be assembled or intersected with other substances in a system to form a three-dimensional network structure, whenwater is encountered, the flow of liquid oil and water in the mixed system is stabilized, thus the overall viscoelasticity is optimized, and the elasticity and taste of the prepared bread are ensured. The composite bread premixed flour solves the problem of insufficient elasticity and poor taste of bread prepared by common bread premixed flour.
Owner:杨晓军
A kind of olanzapine tablet composition and preparation method thereof
ActiveCN104208031BImprove dissolution rateImprove bioavailabilityOrganic active ingredientsNervous disorderMedicineDiluent
The invention provides a new olanzapine tablet composition and a preparation method thereof. The composition selects lactose and microcrystalline cellulose with good fluidity and compression formability as diluents and dry binders, and adopts a process The preparation of simple powder direct compression method has simple process, saves time and energy, improves the disintegration or dissolution speed of the tablet, and also improves the bioavailability of the tablet, and the quality of the prepared olanzapine tablet composition is controllable. And ensure the stability of the product.
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD
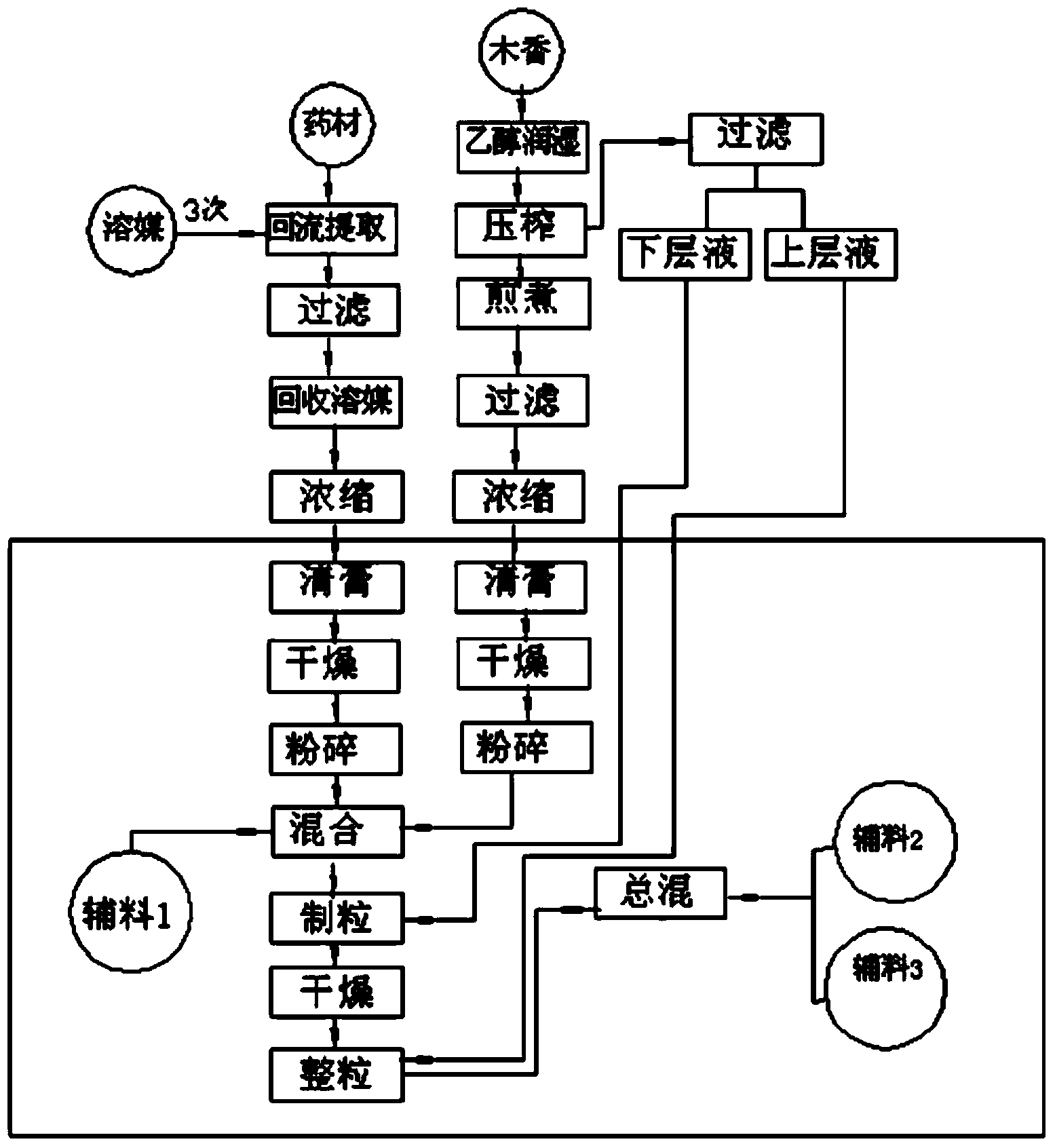
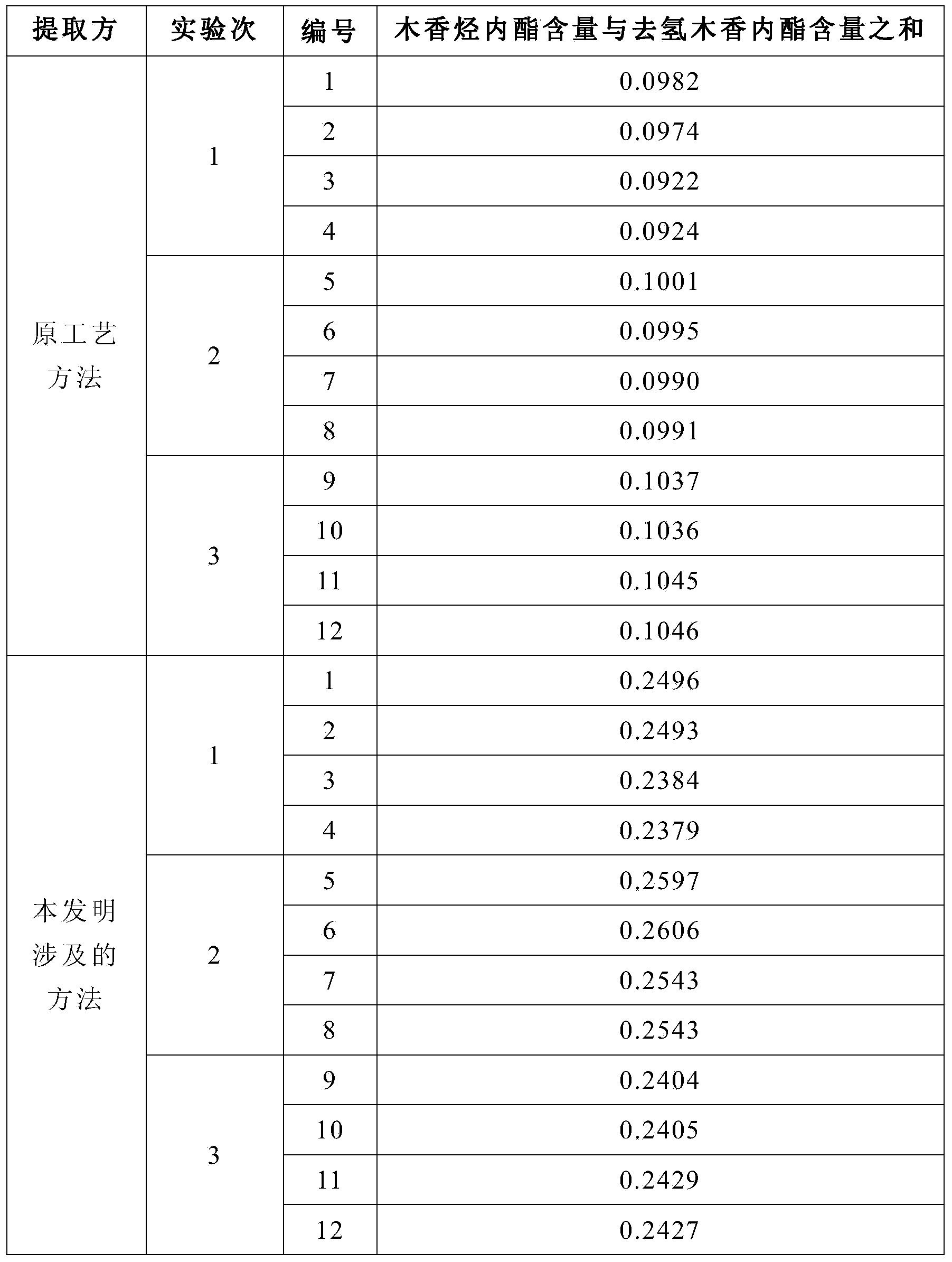
Technology for extracting volatile oil in Radix Aucklandiae, and application of volatile oil in Xianglian tablet
InactiveCN104212640AReduce dosageSolve uneven quality problemsEssential-oils/perfumesPill deliveryMedicineFilter cake
The invention provides a technology for extracting volatile oil in Radix Aucklandiae, and an application of the volatile oil in a Xianglian tablet. The technology comprises the following steps: taking Radix Aucklandiae, carrying out coarse crushing to form 6-8mm particles, storing in a stainless steel container, adding ethanol to immerse the particles, allowing the obtained mixture to stand, sealing, storing for 24h, and stirring during the storage period 3-5 times; and squeezing the immersed Radix Aucklandiae, filtering to obtain a squeezing liquid and a filter cake, allowing the squeezing liquid to stand for 4h in order to obtain a squeezing liquid upper layer and a squeezing liquid lower layer, and separating the squeezing liquid upper layer and the squeezing liquid lower layer for later use. The Xianglian tablet is prepared through wet granulation, the above lower turbid liquid is added during the granulation of the Xianglian tablet, and the above upper clear liquid is added during the granule straightening of the Xianglian tablet. The technology has the advantages of simple flow, easy control and operation, low production device requirements, and low production cost, can be well jointed to a product granulation technology, and effectively guarantees the product quality.
Owner:HUBEI XIANGLIAN PHARMA
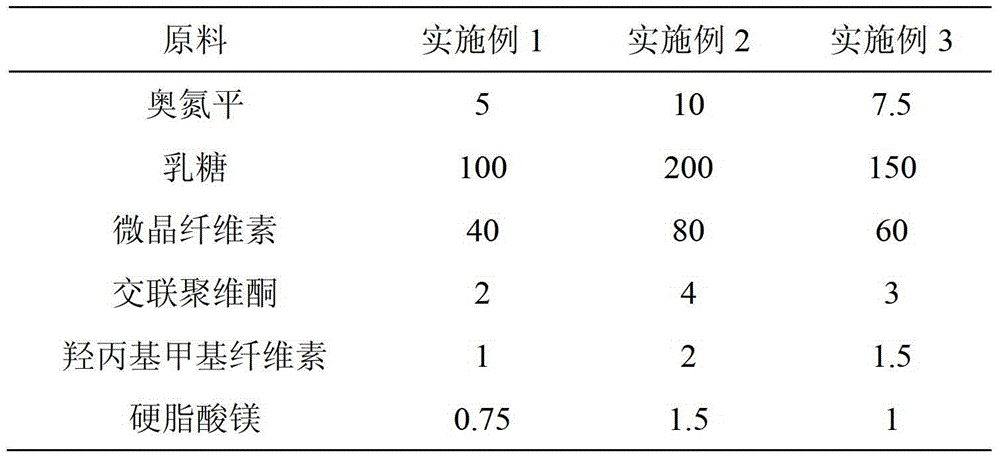
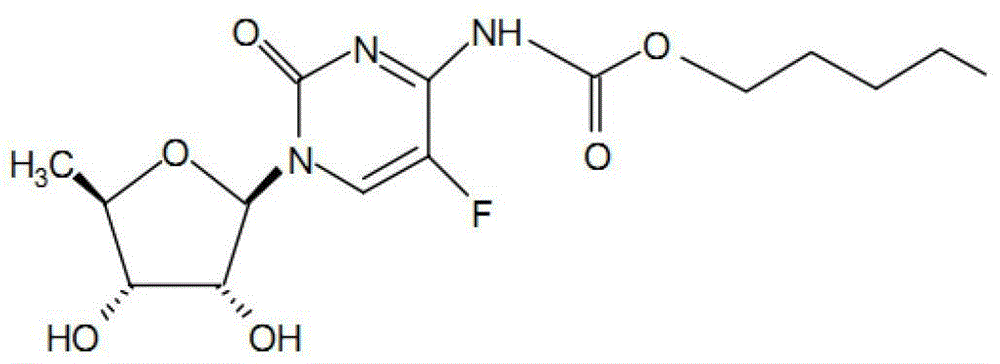
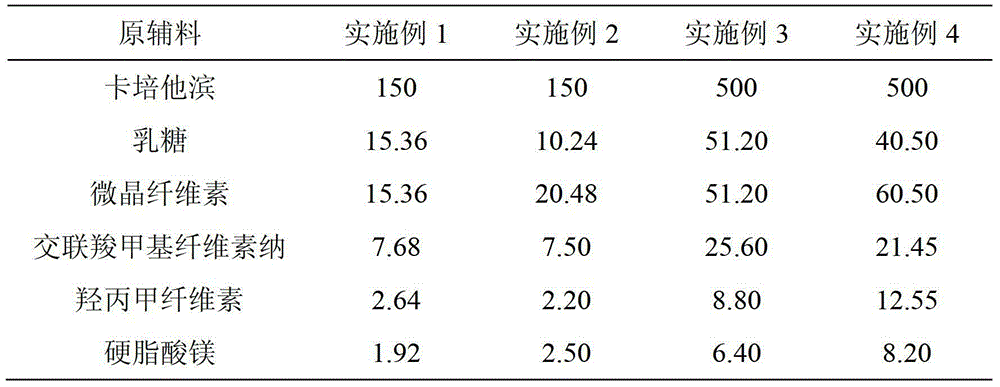
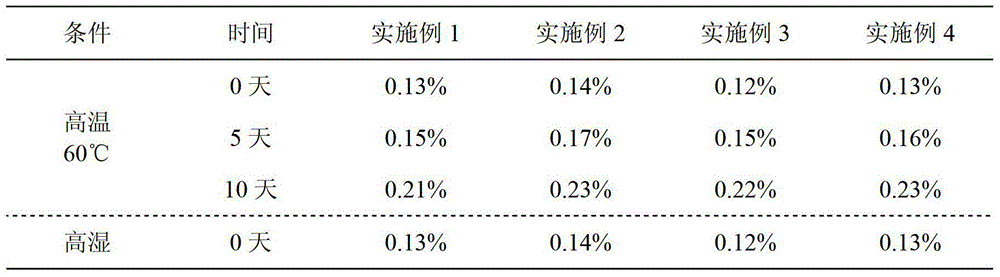
Capecitabine tablet composition and preparation method thereof
ActiveCN103251569BGood compression formabilityUniform particle sizeOrganic active ingredientsPharmaceutical delivery mechanismMedical prescriptionWear resistance
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD
Compound preparation containing capecitabine for treating gastric cancer
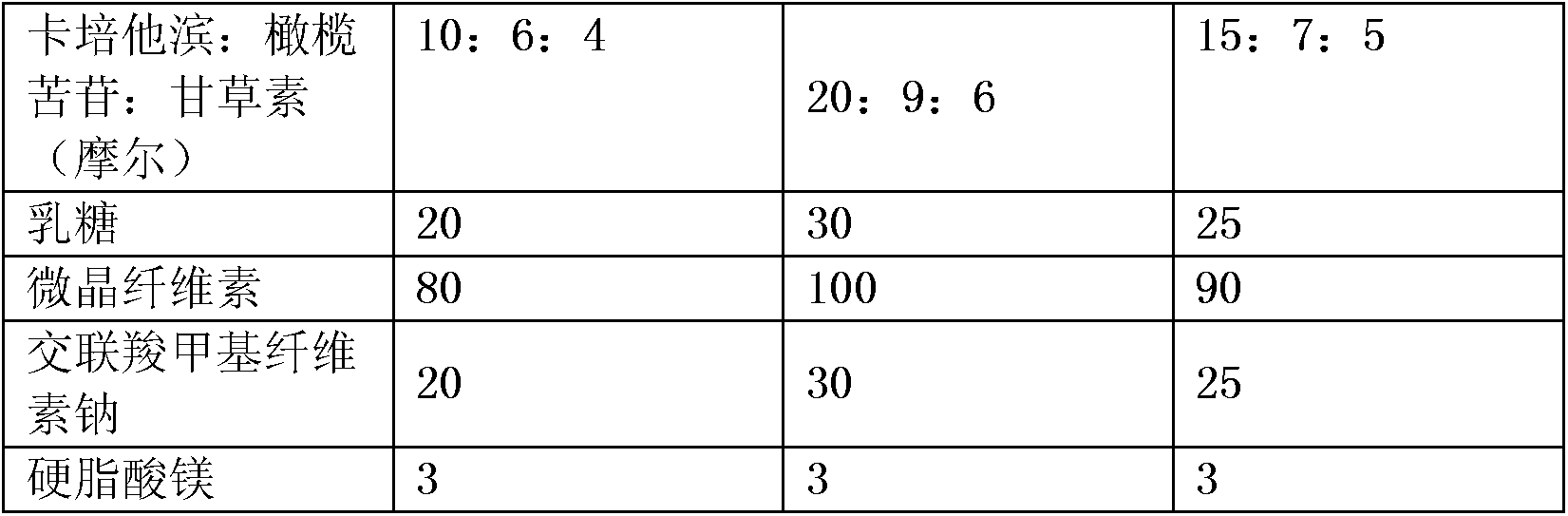
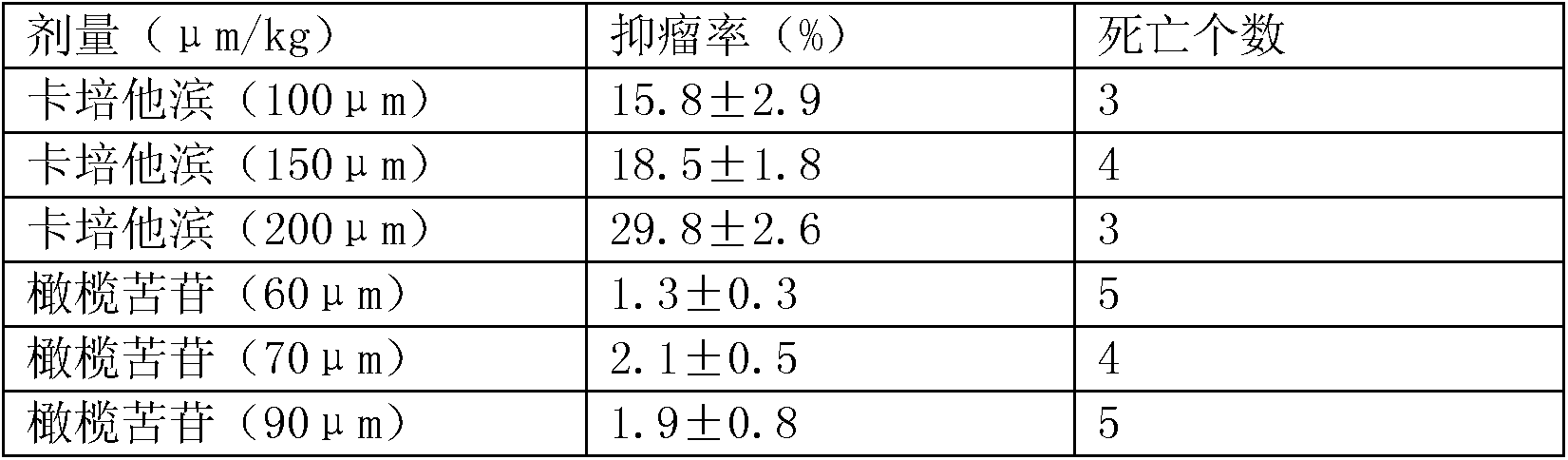
InactiveCN103908464AReduce the amount of treatmentLittle side effectsOrganic active ingredientsDigestive systemLactoseGastric carcinoma
The invention relates to an antitumor drug preparation for treating a gastric cancer. The compound preparation is prepared from a pharmaceutical composition and pharmaceutically acceptable auxiliary materials, wherein the pharmaceutical composition is prepared from capecitabine, oleuropein and glycyrrhizin; the auxiliary materials preferably are lactose, microcrystalline cellulose, croscarmellose sodium, magnesium stearate and the like. Furthermore, the drug preparation is prepared from the following components in parts by weight: 300-500 parts of pharmaceutical composition, 20-30 parts of lactose, 80-100 parts of microcrystalline cellulose, 20-30 parts of croscarmellose sodium and 3 parts of magnesium stearate.
Owner:QINGDAO MUNICIPAL HOSPITAL
Ganoderma sinense polysaccharide dispersible tablet and preparation method thereof
ActiveCN103877039BGood compression formabilityGood disintegrationOrganic active ingredientsNervous disorderGanoderma sinenseWhite blood cell
The invention provides a ganoderma sinense polysaccharide dispersible tablet and a preparation method thereof. The ganoderma sinense polysaccharide dispersible tablet is prepared from the following components in percentage by weight: 20-45% of ganoderma sinense polysaccharide powder, 40-65% of filler, 3-8% of a disintegrating agent, 5-20% of an adhesive, 1.5-3.5% of a corrigent and 0.5-3.0% of a lubricant. The ganoderma sinense polysaccharide dispersible tablet provided by the invention is good in dispersion uniformity and stability, and the technical problems of moisture absorption and sticking in the preparation process are solved. The prepared ganoderma sinense polysaccharide dispersible tablet can be used for treating neurasthenia, leucopenia and thrombopenia as well as symptoms of leucopenia caused by ionizing radiation, occupational hemopoiesis injury and radiotherapy and chemotherapy of tumor patients and the like, and is non-irritant to human body and few in adverse effects.
Owner:江西泽众制药股份有限公司
A kind of preparation method of L-menthol spherical crystal
ActiveCN110002959BSimple processImprove efficiencyOrganic chemistry methodsHydroxy compound separation/purificationMentholActive agent
The invention discloses a preparation method of L-menthol spherical crystals. At 40-60°C, prepare an L-menthol-water mixed solution with an L-menthol concentration of 0.01-0.20g / mL; keep stirring until the liquid-liquid phase separation occurs in the solution; cool the solution to 1-20°C, and continue Stir until crystals appear; add 0.05% to 0.55% surfactant by mass fraction and keep stirring for 0.5 to 5 hours to make the crystals coalesce into balls; filter, wash and dry to obtain L-menthol spherical crystals. The solvent in the crystallization process only involves water, which is green and environmentally friendly, and the process is simple; the particle size of spherical crystals can be adjusted by stirring speed, and the average particle size of the product is between 50 and 300 microns; the crystal particles are round, with high fluidity and compactness The density is 0.45~0.55g / cm 3 ; The product has no tendency to agglomerate and form agglomerates, and can be stored for a long time.
Owner:TIANJIN UNIV
A kind of preparation method of o-vanillin spherical crystal
ActiveCN112194574BSimple processImprove efficiencyCarbonyl compound separation/purificationFluid phaseActive agent
The invention discloses a preparation method of o-vanillin spherical crystals. At 40-50°C, prepare o-vanillin-water mixed suspension with o-vanillin concentration of 0.01-0.20g / mL; keep stirring until liquid-liquid phase separation occurs in the solution; quench the solution to 1-15°C, and continue Stir until the crystals appear; add a surfactant with a mass fraction of 0.02% to 0.40% (based on the mass of the o-vanillin-water mixed solution), and keep stirring for 0.5 to 5 hours to make the crystals coalesce into balls. Filter, wash and dry to obtain o-vanillin spherical crystals. The crystallization process has a single raw material, and the solvent only involves water, which is environmentally friendly and has a simple process. The particle size of spherulite products can be effectively adjusted by the stirring rate. The average particle size of the product is about 300-1200 microns; the crystal particles are round, with high fluidity, the angle of repose is between 30°-33°, and the tap density is 0.50-0.58 g / cm 3 .
Owner:TIANJIN UNIV
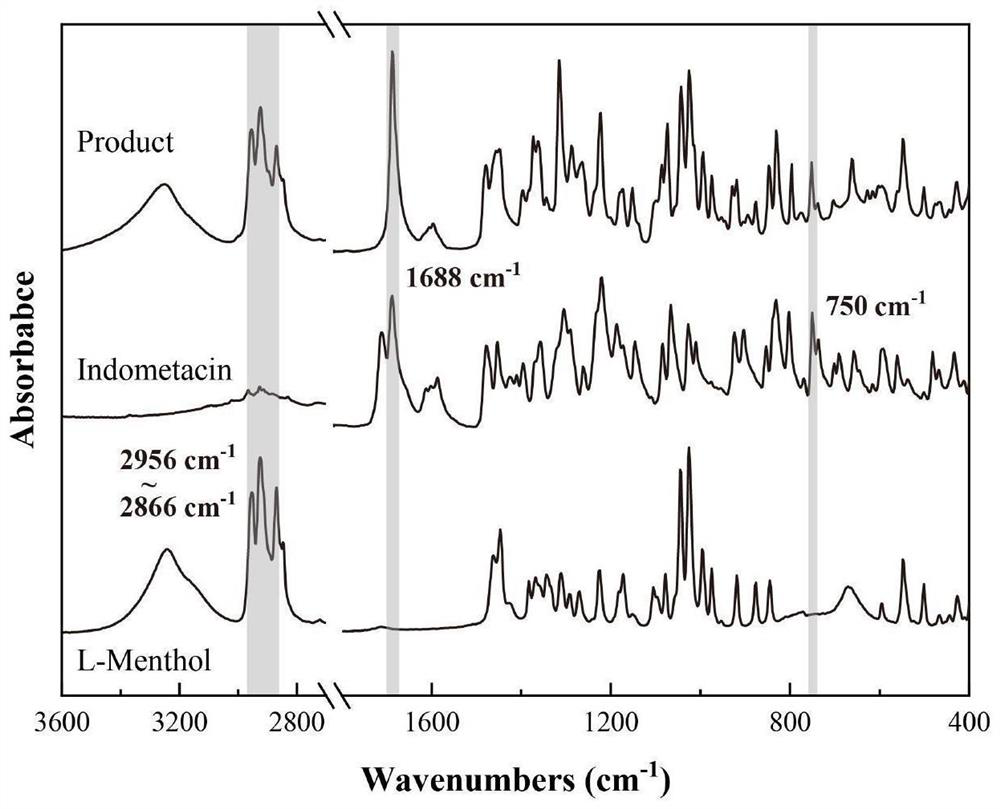
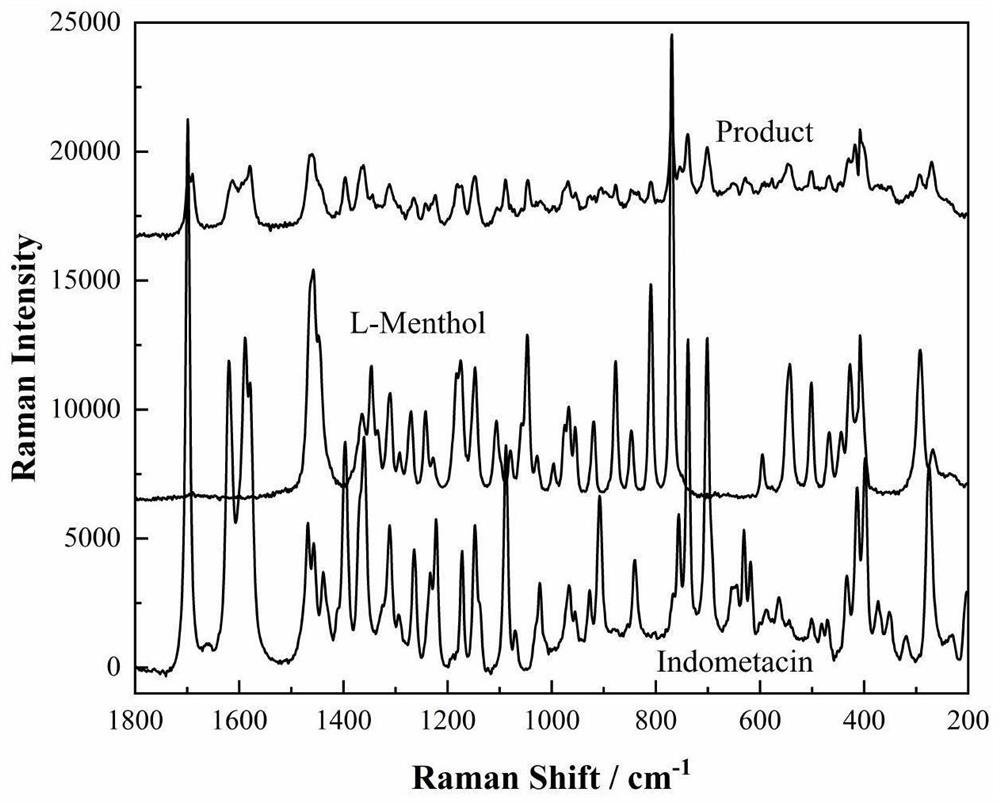
L-menthol loaded indomethacin composite particle and preparation method thereof
ActiveCN112972390AMeet the requirements for combination medicationImprove efficacyPowder deliveryHydroxy compound active ingredientsIndometacinMenthol
The invention provides an L-menthol loaded indomethacin composite particle and a preparation method thereof. The composite particle is spherical, and the mass ratio of L-menthol to indomethacin in the composite particle is (0.63-3.55): 1. The composite particle has good uniformity and liquidity, and is not easy to coalesce and adjustable in proportion between components in a product. The L-menthol exists in a stable alpha crystal form, and the indomethacin exists in a medical gamma crystal form. The composite particle can significantly increase percutaneous rates so as to enhance treatment effects. The preparation method of the composite particle is simple, low in cost, easy to realize without using ingredients, round in particle, low in energy consumption and high in efficiency; the composite particle merely uses water as a solvent, so that the composite particle is green and environment-friendly, and can be highly mixed, therefore industrialization and low economic investment can be realized.
Owner:TIANJIN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com