Indissolvable drug oral sustained-release dry emulsion tablet and preparation method thereof
A technology of insoluble drugs and dry milk tablets, which is applied in the fields of pharmaceutical formula, drug combination, drug delivery, etc., can solve the problems of short half-life, short duration of drug effect, prolonged duration of drug effect, peak and valley phenomenon of blood drug concentration, etc. Achieve the effects of reducing the first-pass effect of the liver, taking less times, and improving oral bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
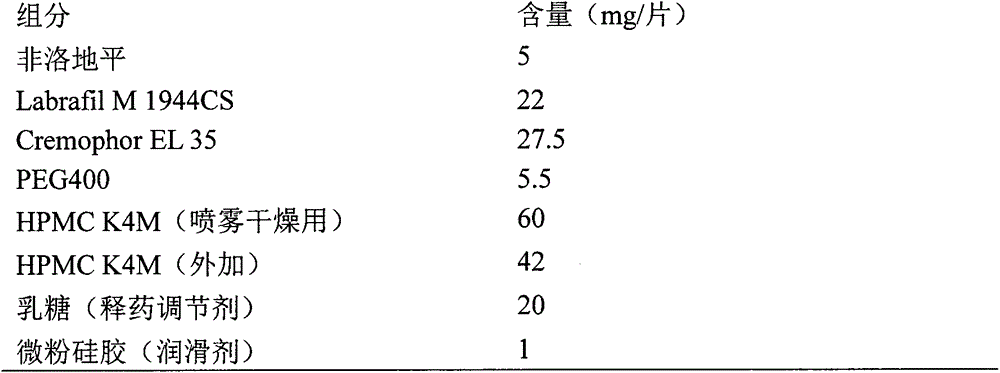
[0042] Prescription composition:
[0043]
[0044] 3% PVPK30 in 70% ethanol solution as adhesive, made 1000 pieces in total
[0045] Preparation Process:
[0046] 1) The drug is completely dissolved in the self-emulsifying system to obtain the drug-loaded self-emulsifying system;
[0047] 2) adding a small amount of water to the drug-loaded self-emulsifying system to make it self-emulsified, and then mixing it with an aqueous solution of HPMCK4M for spray drying to obtain a material liquid for spray drying;
[0048] 3) spray drying;
[0049] 4) Sieve and mix dry milk powder with extra HPMC and lactose, use 3% PVPK30 in 70% ethanol solution as binder, sieve and granulate at 20 mesh, dry at 45°C for 4 hours, granulate at 20 mesh, and finally add lubricant Tablet.
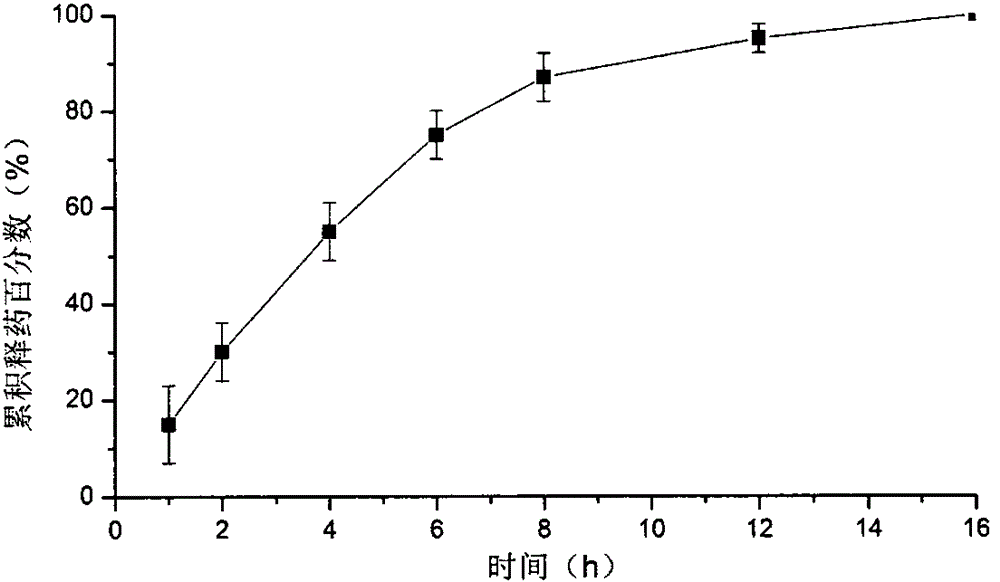
[0050] The obtained felodipine oral sustained-release dry milk tablet was tested for release according to the following dissolution method. Its drug release curve is shown in figure 1 .
[0051] According to...
Embodiment 2
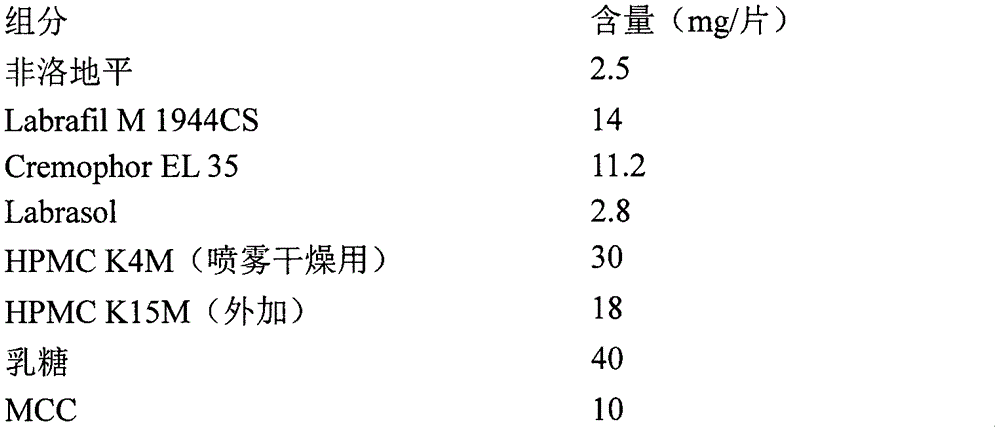
[0053] Prescription composition:
[0054]
[0055]
[0056] A 70% ethanol solution of 3% PVPK30 was used as an adhesive to make a total of 1000 dosage units. The preparation process was the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com