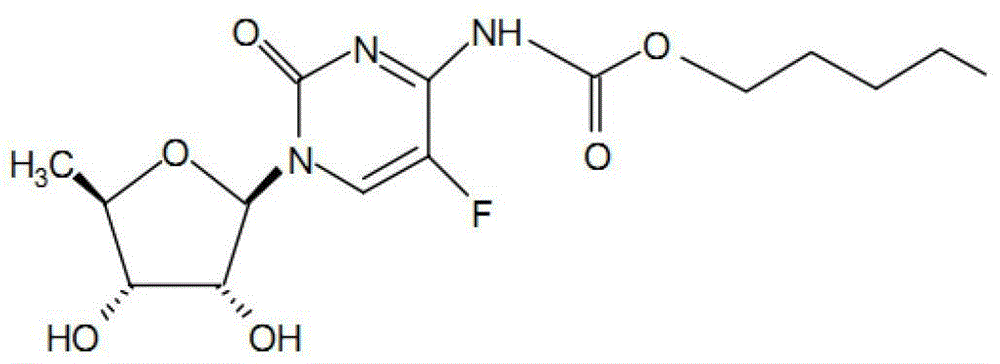
Capecitabine tablet composition and preparation method thereof
A technology of capecitabine and its composition, which is applied in the field of capecitabine tablet composition and its preparation, can solve the problems that it is not suitable for making dispersible tablets, and achieve the effects of qualified quality, reduced production process, and good feasibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
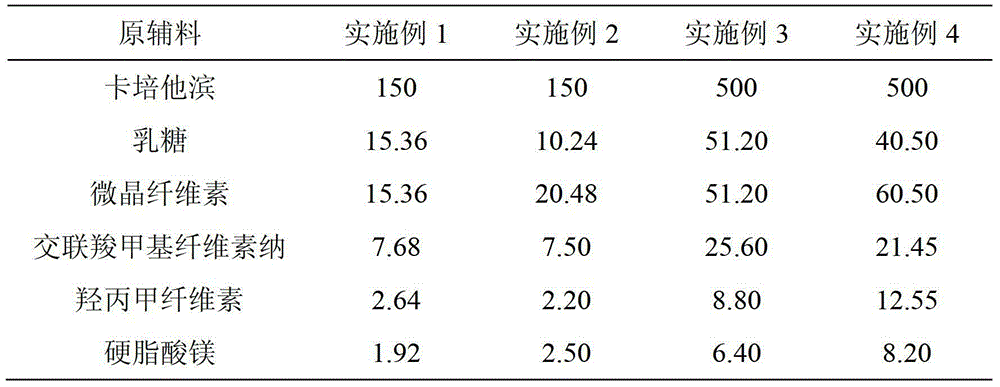
Embodiment 1
[0032] Preparation Process:
[0033] (1) Ingredients: Weigh the prescribed amount of capecitabine, lactose, microcrystalline cellulose, and croscarmellose sodium, pass the capecitabine raw materials through an 80 mesh screen, and weigh the prescribed amount of hydroxypropyl Methyl cellulose, prepared into a 2.5% (w / w) aqueous solution for use;
[0034] (2) Pre-mixing: mix the capecitabine, lactose, microcrystalline cellulose weighed in step (1) and 3 / 4 prescription amount of croscarmellose sodium;
[0035] (3) Granulation: Put the mixed material in a wet granulator, stir for 3~5min, add 2.5% (w / w) hypromellose aqueous solution to the material to make wet granules after the stirring is complete, and the slurry is completed After that, turn on the shearing knife, stir and cut, and use a granulator to wet granulate;
[0036] (4) Drying: Put the wet particles into the drying equipment, the inlet air temperature is not higher than 80℃, and the moisture content is controlled to be less tha...
Embodiment 2
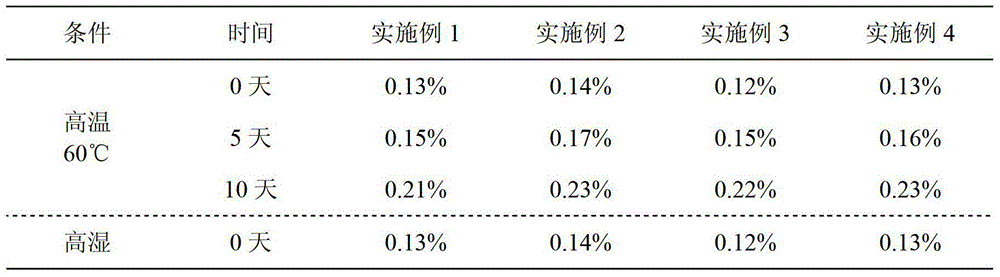
[0042] Preparation process: same as the preparation process of Example 1, the hardness of the tablet is controlled within 50-70N, and the disintegration time limit is less than 7 minutes.
Embodiment 3
[0044] Preparation process: same as the preparation process of Example 1, the hardness of the tablet is controlled within 60-80N, and the disintegration time limit is less than 13 minutes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com