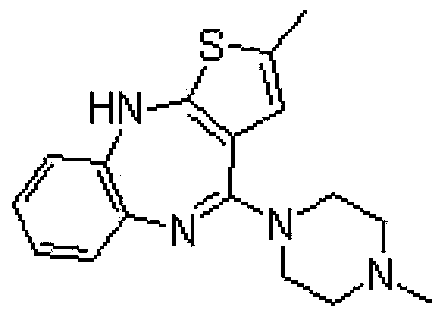
Olanzapine tablet composition and preparation method thereof
A technology for olanzapine tablet and composition, applied in the field of olanzapine tablet composition and preparation thereof, can solve the problems of poor powder fluidity, large difference in tablet weight, powder splinter, etc., and achieves the elimination of poor fluidity, qualified quality and properties stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
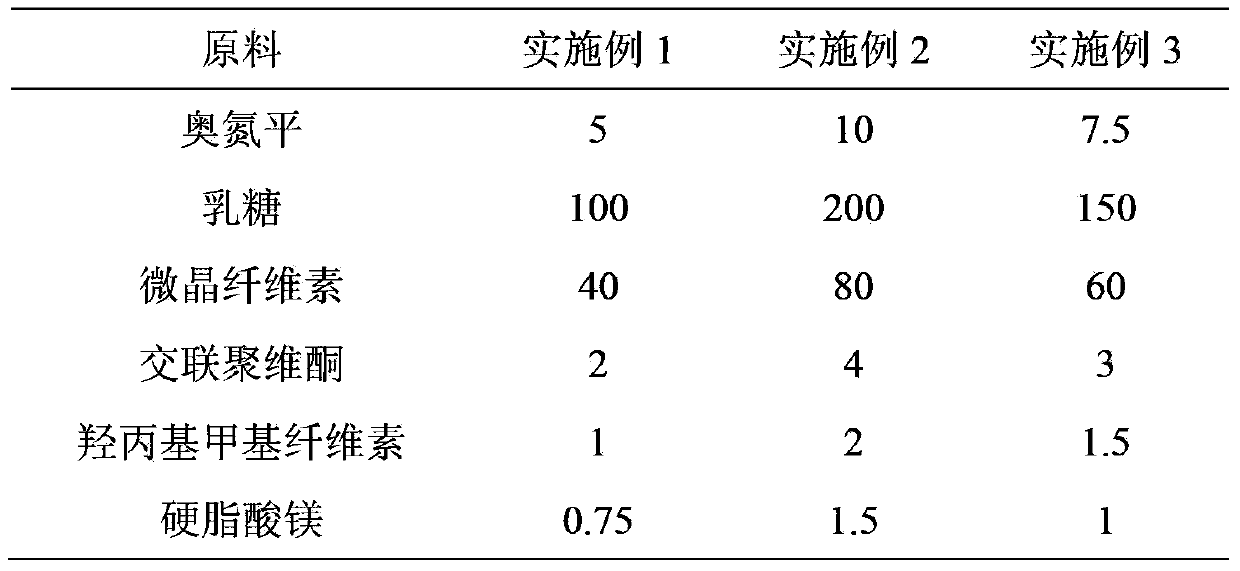
Embodiment 1
[0026] Preparation Process:
[0027] (1) Ingredients: Weigh the prescribed amount of olanzapine, lactose, microcrystalline cellulose, crospovidone, hydroxypropyl methylcellulose, magnesium stearate, crush and sieve the raw materials if necessary, Make it fit 100 mesh sieve;
[0028] (2) Mixing: Mix lactose, crospovidone, olanzapine, microcrystalline cellulose and hydroxypropyl methylcellulose for 40 minutes to make them uniform;
[0029] (3) Add the prescribed amount of magnesium stearate to the powder mixed uniformly in step (2), and mix for 10 minutes to make it uniform;
[0030] (4) Press the uniformly mixed medicinal powder in step (3) into tablets directly, and control the hardness at 50N-75N;
[0031] (5) Coating: Add the prescribed amount of film coating premix Opadry 85G68918 into the stirring water, and stir evenly to obtain a 20% (w / w) coating solution for later use. Take the vegetarian tablet and put it in the coating pan, control the pan speed to 5-10 revolution...
Embodiment 2
[0033] Preparation process: same as the preparation process of Example 1, mixing in step (2) for 20 minutes to make it uniform, and controlling the hardness at 55N-80N.
Embodiment 3
[0035] Preparation process: same as the preparation process of Example 1, mixing in step (2) for 30 minutes to make it uniform, and controlling the hardness at 50N-80N. Comparative example: According to the prescription and preparation process of 5 mg olanzapine per tablet disclosed in Example 2 of Chinese Patent No. ZL96192778.X, a comparative example sample was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com