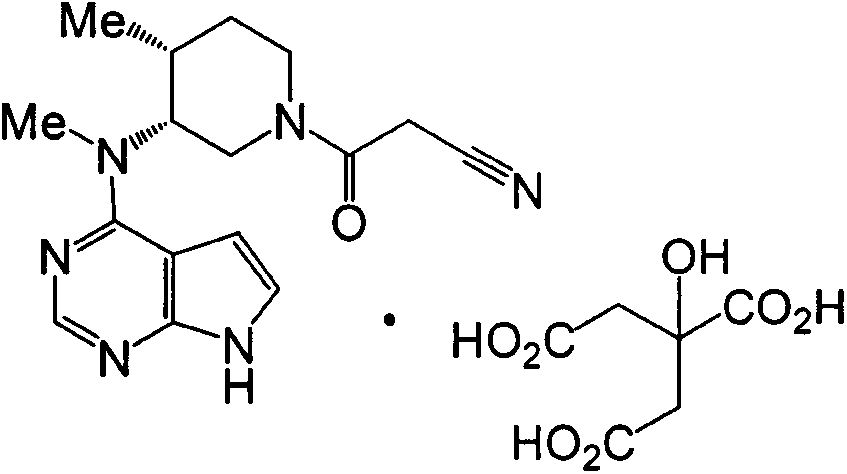
Tofacitinib tablet with excellent property
A tofacitinib and tablet technology, applied in the field of tofacitinib tablets, can solve problems such as the inability to meet the needs of patients with rheumatoid arthritis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
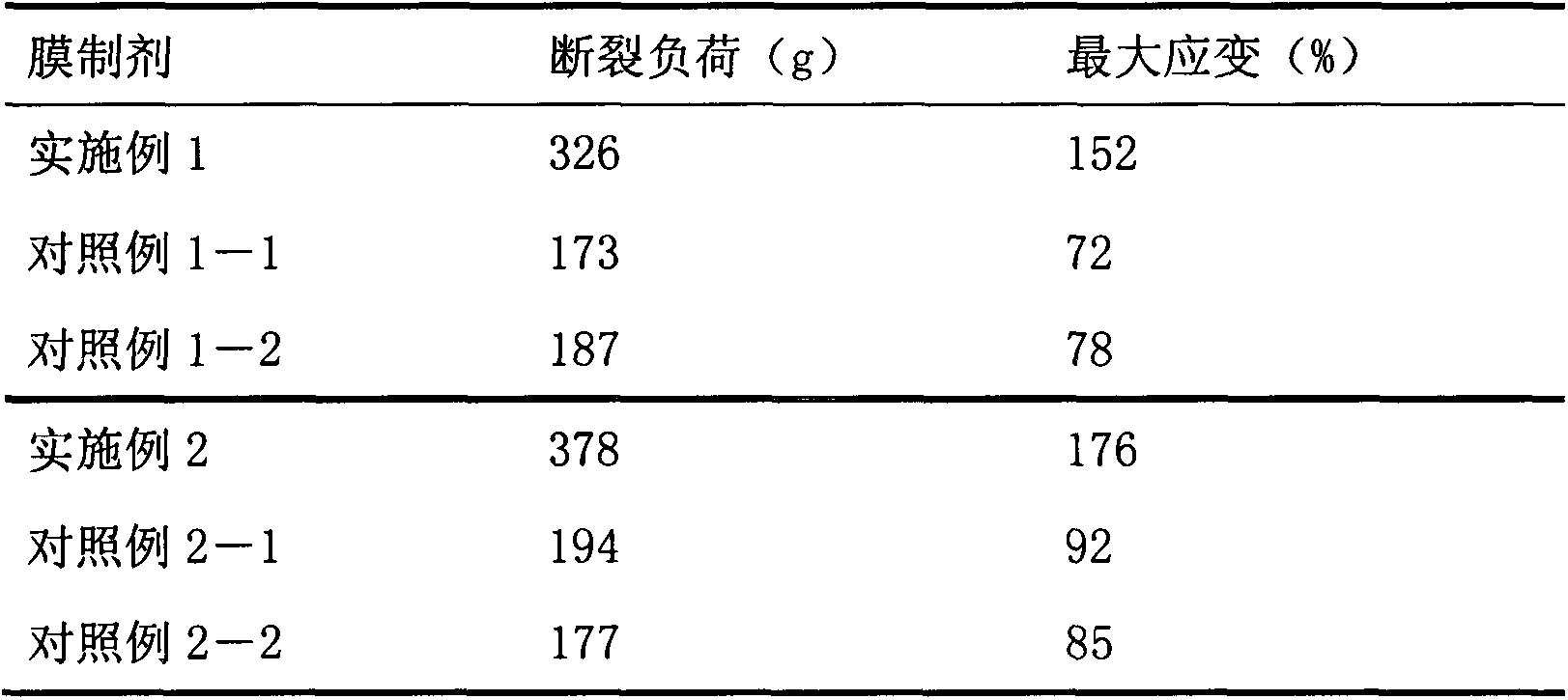
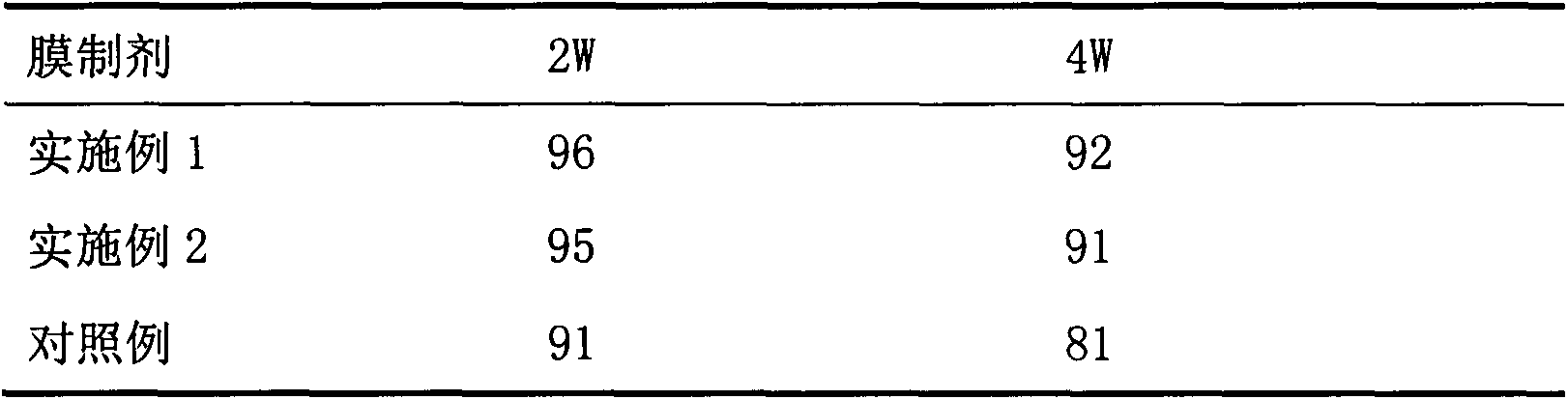
Embodiment 1
[0043] Tofacitinib (160g), hydroxypropyl cellulose (150.0g), low-substituted hydroxypropyl cellulose (400.0g), lactose (1090g) and crystalline cellulose (200.0g) passed The high intensity mixer was mixed for 3 minutes, then magnesium stearate (20.0 g) was added, and the mixture was mixed again using the high intensity mixer to obtain a mixed powder.
[0044] The obtained mixed powder was compressed by using a rotary compressor under a tablet pressure of 6.9 kN so that the tablet mass became about 100 mg and the thickness was not more than 2 mm. The obtained uncoated tablet was sprayed with a mixture of polyvinyl alcohol (OPADRYAMB31W48994 (manufactured by Colorcon (Japan) Limited)), pullulan and sorbitol (wherein, polyvinyl alcohol: pullulan: sorbitol Sugar alcohol=1:1:0.2) and the coating solution that water forms, carry out film-coating in the disc coater, obtain the tablet (coating film gain is 10 to 40% of tablet mass) that contains test compound.
Embodiment 2
[0046] Tofacitinib (160g), hydroxypropyl cellulose (150.0g), low-substituted hydroxypropyl cellulose (400.0g), lactose (1090g) and crystalline cellulose (200.0g) passed The high intensity mixer was mixed for 3 minutes, then magnesium stearate (20.0 g) was added, and the mixture was mixed again using the high intensity mixer to obtain a mixed powder.
[0047] The obtained mixed powder was compressed by using a rotary tablet press under a tablet pressure of 6.9 kN so that the tablet mass became about 100 mg and the thickness was not more than 1 mm. The obtained uncoated tablet was sprayed with a mixture of polyvinyl alcohol (OPADRYAMB31W48994 (manufactured by Colorcon (Japan) Limited)), pullulan, PEG1250 (wherein, polyvinyl alcohol: pullulan: PEG1250=1 : 5: 0.5) and water, were coated in a disc coater to obtain tablets containing the test compound (the coating gain was 30 to 1000% of the mass of the tablet).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com